Abstract
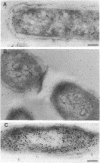
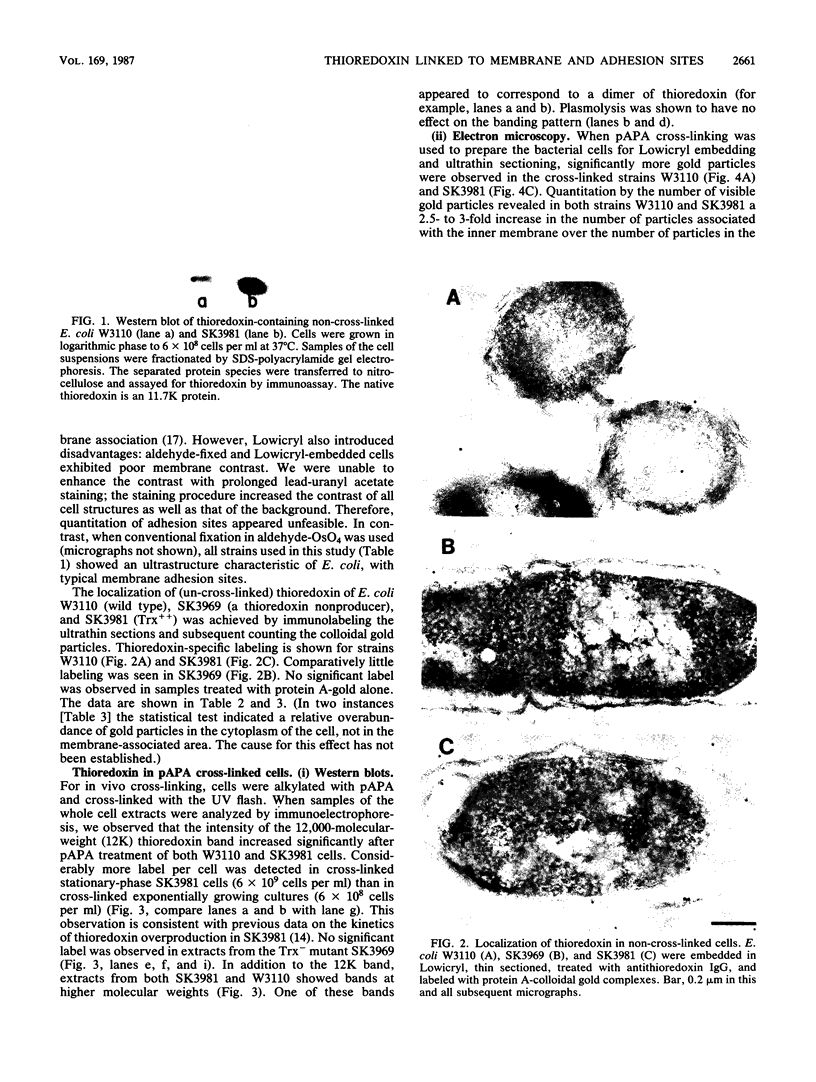
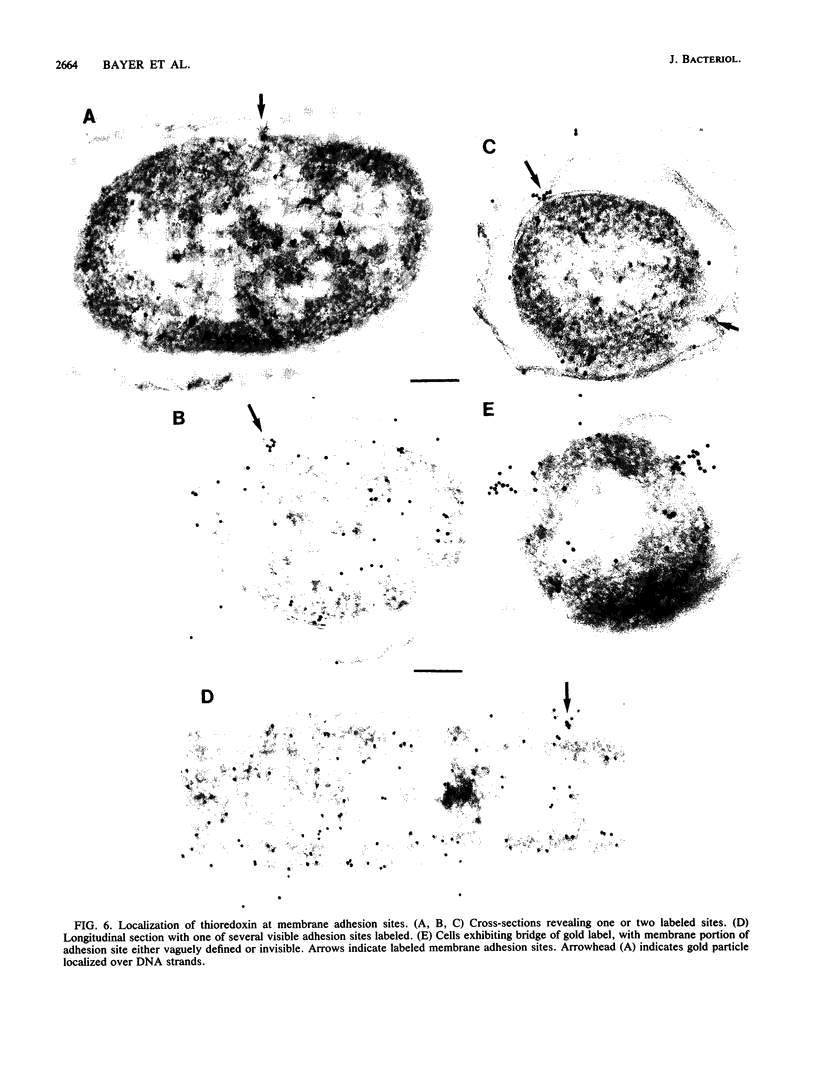
The intracellular localization of thioredoxin in Escherichia coli was determined by immunoelectron microscopy and correlated to previous biochemical data which had suggested that thioredoxin resides at inner-outer membrane adhesion sites. Since a considerable amount of thioredoxin was lost during preparation of cells for electron microscopy, we immobilized the protein with the heterobifunctional photoactivatable cross-linker p-azidophenacylbromide before the cells were fixed with aldehyde and embedded in Lowicryl K4M. Thin sections were labeled with affinity-purified antithioredoxin antiserum and protein A-gold complexes. Densities of immunolabel in a designated membrane-associated area and in the rest of the cytoplasm were compared and the data were statistically evaluated. Wild-type strain W3110 and strain SK3981, an overproducer of thioredoxin, exhibited increased labeling at the inner membrane and its adjacent cytoplasmic area. In contrast, the more centrally located cytoplasm of both strains showed much lower label density. This label distribution did not change with cell growth or in the stationary phase. Immunolabel was often found at bridges between the inner and outer membranes; this result is consistent with a model which places at least a portion of the thioredoxin at membrane adhesion sites, corresponding to an osmotically sensitive cytoplasmic compartment bounded by a hybrid inner-outer membrane (C.A. Lunn and V. Pigiet, J. Biol. Chem. 257:11424-11430, 1982; C.A. Lunn and V. Pigiet, J. Biol. Chem. 261:832-838, 1986). Specific label was absent in the periplasmic space.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S., Modrich P. T7-induced DNA polymerase. Requirement for thioredoxin sulfhydryl groups. J Biol Chem. 1983 Jun 10;258(11):6956–6962. [PubMed] [Google Scholar]
- Bayer M. E., Bayer M. H. Effects of bacteriophage fd infection on Escherichia coli HB11 envelope: a morphological and biochemical study. J Virol. 1986 Jan;57(1):258–266. doi: 10.1128/jvi.57.1.258-266.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. H., Bayer M. E. Phosphoglycerides and phospholipase C in membrane fractions of Escherichia coli B. J Bacteriol. 1985 Apr;162(1):50–54. doi: 10.1128/jb.162.1.50-54.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. H., Costello G. P., Bayer M. E. Isolation and partial characterization of membrane vesicles carrying markers of the membrane adhesion sites. J Bacteriol. 1982 Feb;149(2):758–767. doi: 10.1128/jb.149.2.758-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Hobot J. A., Villiger W., Escaig J., Maeder M., Ryter A., Kellenberger E. Shape and fine structure of nucleoids observed on sections of ultrarapidly frozen and cryosubstituted bacteria. J Bacteriol. 1985 Jun;162(3):960–971. doi: 10.1128/jb.162.3.960-971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Lopez J., Webster R. E. Assembly site of bacteriophage f1 corresponds to adhesion zones between the inner and outer membranes of the host cell. J Bacteriol. 1985 Sep;163(3):1270–1274. doi: 10.1128/jb.163.3.1270-1274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn C. A., Kathju S., Wallace B. J., Kushner S. R., Pigiet V. Amplification and purification of plasmid-encoded thioredoxin from Escherichia coli K12. J Biol Chem. 1984 Aug 25;259(16):10469–10474. [PubMed] [Google Scholar]
- Lunn C. A., Pigiet V. P. Chemical cross-linking of thioredoxin to hybrid membrane fraction in Escherichia coli. J Biol Chem. 1986 Jan 15;261(2):832–838. [PubMed] [Google Scholar]
- Lunn C. A., Pigiet V. P. Localization of thioredoxin from Escherichia coli in an osmotically sensitive compartment. J Biol Chem. 1982 Oct 10;257(19):11424–11430. [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. Goblin (ankyrin) in striated muscle: identification of the potential membrane receptor for erythroid spectrin in muscle cells. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3292–3296. doi: 10.1073/pnas.81.11.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren H., Rozell B., Holmgren A., Hansson H. A. Immunoelectron microscopic localization of glutaredoxin and thioredoxin in Escherichia coli cells. FEBS Lett. 1981 Oct 12;133(1):145–150. doi: 10.1016/0014-5793(81)80492-9. [DOI] [PubMed] [Google Scholar]
- Pollitt S., Zalkin H. Role of primary structure and disulfide bond formation in beta-lactamase secretion. J Bacteriol. 1983 Jan;153(1):27–32. doi: 10.1128/jb.153.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase. EMBO J. 1984 Oct;3(10):2393–2397. doi: 10.1002/j.1460-2075.1984.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Thioredoxin is required for filamentous phage assembly. Proc Natl Acad Sci U S A. 1985 Jan;82(1):29–33. doi: 10.1073/pnas.82.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G., Jacoby R. Conformational changes associated with proteolytic processing of presecretory proteins allow glutathione-catalyzed formation of native disulfide bonds. J Biol Chem. 1982 Oct 25;257(20):12277–12282. [PubMed] [Google Scholar]