Abstract
The brain demands oxygen and glucose to fulfill its roles as the master regulator of body functions as diverse as bladder control and creative thinking. Chemical and electrical transmission in the nervous system is rapidly disrupted in stroke as a result of hypoxia and hypoglycemia. Despite being highly evolved in its architecture, the human brain appears to utilize phylogenetically conserved homeostatic strategies to combat hypoxia and ischemia. Specifically, several converging lines of inquiry have demonstrated that the transcription factor hypoxia-inducible factor-1 (HIF1-1) mediates the activation of a large cassette of genes involved in adaptation to hypoxia in surviving neurons after stroke. Accordingly, pharmacological or molecular approaches that engage hypoxic adaptation at the point of one of its sensors (e.g., inhibition of HIF prolyl 4 hydroxylases) leads to profound sparing of brain tissue and enhanced recovery of function. In this review, we discuss the potential mechanisms that could subserve protective and restorative effects of augmenting hypoxic adaptation in the brain. The strategy appears to involve HIF-dependent and HIF-independent pathways and more than 70 genes and proteins activated transcriptionally and post-transcriptionally that can act at cellular, local, and system levels to compensate for oxygen insufficiency. The breadth and depth of this homeostatic program offers a hopeful alternative to the current pessimism towards stroke therapeutics.
Keywords: Brain, Stroke, Hypoxia, HIF, HIF prolyl hydroxylase, Therapeutics
Stroke is defined as injury to the brain accruing from a vascular etiology. Strikingly, it has emerged as the third leading cause of death and the leading cause of disability in the USA. Accordingly, the estimated financial costs of stroke are more than 50 billion dollars a year in the USA alone. These financial costs do not begin to tell the story of the personal suffering that amasses from the silent epidemic of stroke disability—over 5 million Americans face the challenges of handicaps from stroke each day. The recognition of stroke as a leading age-associated public health issue has led the government and the pharmaceutical industry to expend enormous resources on developing interventions in the form of drugs that minimize brain damage associated with stroke. Despite their promise, these efforts have been disappointing and have left a nearly indelible sense of frustration on the biomedical research community [1]. In this chapter, we will discuss the pathophysiology of stroke with particular attention to one of its primary mediators, hypoxia, and attempt to revive a sense of optimism and enthusiasm for stroke therapeutics moving forward.
Ischemia is a process in which perfusion to tissue is critically reduced creating a deficit in necessary brain fuels. The brain is highly vulnerable to ischemia because the eloquent functions it is assigned to carry out, in the pluralistic society of organ functions, depend integrally on energy—specifically adenosine triphosphate (ATP). The brain comprises only 2% of body weight, but it utilizes nearly 20% of cardiac output to achieve its supply of essential nutrients including oxygen and glucose. Abnormal central nervous system (CNS) symptoms begin to manifest at 40–50% of normal resting hemispheric cerebral blood flow resulting in slowing of the EEG, attenuation of evoked potentials, and reduction in the membrane potential in individual cortical neurons [2]. The resting membrane potential must be maintained to allow proper neuronal function, including synaptic activity and axonal conduction. Once the neuronal membrane potential begins to dissipate, neuronal function ceases. It is important to note that ATP levels at this point may be normal or only slightly reduced [3]. The reason for this inordinate sensitivity is not entirely clear but is likely related to the Km of neurotransmitter systems for ATP or their exquisite sensitivity to tissue acidosis [4]. Gross deterioration and damage requires even more severe reductions in blood flow to 20–30% of normal resting hemispheric cerebral blood flow (Fig. 1). With this level of ischemia, a deterioration of ionic membrane gradients ensues, and the tissue begins to accumulate hydrogen ions (acidosis) [5]. Changes in ionic fluxes likely accrue directly from a reduction of intracellular ATP, a failure of the Na+/K+ATPase activity, and increases in intracellular Na+ and extracellular K+. The failure of ionic homeostasis reflects loss of activity of multiple transporters that normally maintain the electrochemical gradients necessary for normal neuronal signal transduction [6, 7]. Among the ions deregulated, intracellular calcium appears to play a critical role in further ion dyshomeostasis via the calmodulin-dependent activation of neuronal nitric oxide synthase (nNOS) [8–10]. Increased nNOS activity leads to increase in the ambient levels of neuronal nitric oxide (NO) [11]. Changes in NO coupled with ischemia-associated increases in cytosolic and mitochondrially derived superoxide, combine to form toxic peroxynitrite. Peroxynitrite can trigger cell death pathways via DNA damage, poly(ADP-ribose)polymerase (PARP) activation, liberation of apoptosis-inducing factor (AIF) from the mitochondria, and activation of death signaling pathways leading to apoptosis [12, 13]; alternatively, non-selective cation channels such as TRPM2 and TRPM7 can be activated to ensure sustained calcium overload and death [14]. Extracellular acidosis is also postulated to activate acid sensing ion channels, which also contribute to destruction of the normal ionic environment [15]. As one can appreciate from a partial description of the sequence of events after stroke that occur in the neuron alone, targeting a single molecule in the complex parallel and serial pathways of acute hypoxia-ischemia will not maintain neuronal survival (Fig. 2). It is also unlikely to enhance the ability of energy-thirsty neurons to carry out their sophisticated roles in maintaining posture, movement, sequencing language, or making critical executive decisions. Therefore, how might we move forward? One of the most important advances in the treatment of complex medical problems has been the discovery that multimodal therapies can greatly enhance therapeutic efficiency. Treatment of cancer, HIV infection, and tuberculosis with multimodal therapies yields results that are not obtained with the application of single therapeutic agents.
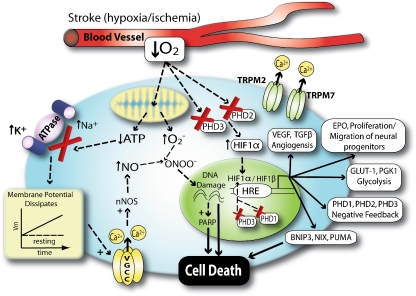
Fig. 1.
Neuronal hypoxia leads to calcium overload and production of free radicals. Stroke is associated with a decrease in cerebral blood flow to the brain. The consequent loss of metabolic fuels leads to failure of sodium pumps leading to an intracellular accumulation of sodium and calcium, depolarization, and activation of voltage sensitive and ligand gated [N-methyl-d-aspartate (NMDA)] ion channels. Increased in calcium in microdomains near the NMDA receptor leads to activation of neuronal nitric oxide synthase. Global dysregulation of calcium in the neuron leads to mitochondrial overload and superoxide production. Nitric oxide and superoxide combine to form peroxynitrite. Peroxynitrite can damage DNA leading to PARP activation and consumption of NAD+. It can also activate TRPM2/7 channels leading to further calcium dysregulation. Hypoxia is sensed by decreased activity of HIF prolyl 4 hydroxylases that can lead to activation genetic responses capable of compensating for the sentinel metabolic stress (decreased cerebral blood flow). Decrease HIF PHD activity can also prevent death via HIF-independent pathways. Acidosis and ROS can also combine with HIF regulated prodeath proteins to trigger cell death
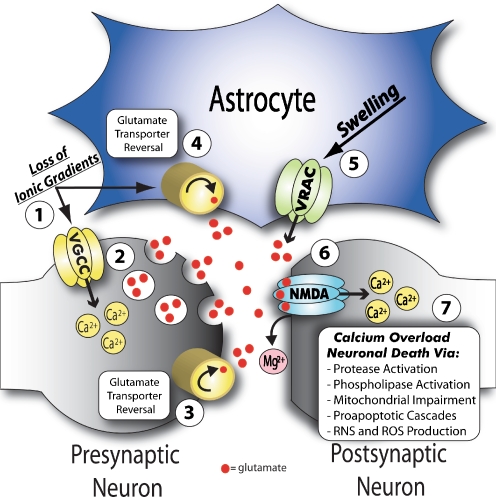
Fig. 2.
Contribution of neurons and astrocytes in mediating excitotoxic neuronal death. 1 Loss of ATP in ischemia leads to inhibition of the Na+/K+ATPase and subsequent collapse of normal ionic gradients. 2 In turn, neuronal membrane depolarization activates voltage sensitive Ca2+ channels, which increase intracellular Ca2+ and stimulate vesicular glutamate release. Severe loss of ionic gradients found in certain ischemic regions may also lead to the reversal of 3 neuronal specific and 4 astrocyte specific glutamate transporters, which in the reverse mode act to release glutamate into the extracellular space. 5 Cell swelling in cerebral ischemia, which is mainly localized to astrocytes, likely activates swelling sensitive anion channels, referred to as volume regulated anion channels (VRACs). VRACs, which are permeable to organic osmolytes, contribute to glutamate release predominantly in the ischemic penumbra. 6 Glutamate regulated NMDA receptors (NMDA-R) are activated by (1) extracellular glutamate and (2) release of Mg2+ from its pore after membrane depolarization (in part due to activation of glutamate regulated AMPA receptors, not shown). 7 NMDA-Rs are permeable to Ca2+ and as such, overabundant NMDA-R activation leads to an intracellular Ca2+ overload. This increase in intracellular Ca2+ then contributes to neuronal death via several mechanisms
As stroke does not represent a single homogeneous category of injury, it is also a poor candidate for a single approach to treatment [16]. Challenges involved in promoting recovery from stroke involve reducing the extent of damage that occurs in acute injury. Moreover, many different kinds of damage are found in individuals with stroke ranging from necrotic, apoptotic, or parthanatotic death of neurons [17, 18], demyelination of otherwise functional axons [19], and transection of axons and subsequent loss of critical neuronal populations [20]. Acute injury itself is extremely complex, including waves of cell death, inflammatory responses, edema, and scarring. Indeed, experimental studies have continued to demonstrate that interventions that target single aspects of the complex cascade, including blocking ion gradients, scavenging free radicals, or enhancing growth factors on their own are insufficient to overcome the considerable barriers to protection against hypoxia and ischemia in acute stroke [21].
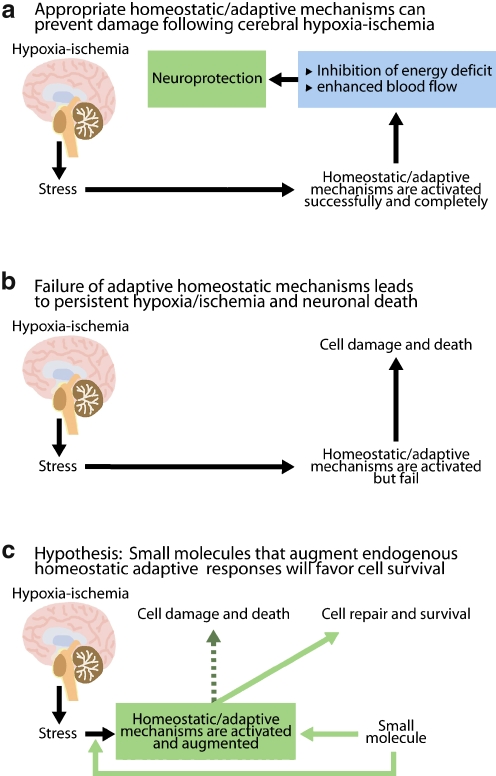
Instructive data from the experimental paradigm of ischemic preconditioning has pointed the way towards novel strategies that can address the heterogeneity and complexity inherent in stroke pathophysiology [22]. Animals subjected to a non-lethal exposure to hypoxia are found to be more resistant to a host of subsequent lethal stresses, including cerebral ischemia. The mechanism by which a sublethal exposure to hypoxia can render the brain resistant to cerebral ischemia and a host of other insults is a topic of active investigation and debate. However, one point appears irrefutable: The tolerance that develops after a short duration of hypoxia involves not only the activation or inactivation of pre-existing proteins but also de novo gene expression [23, 24]. These transcriptional and post-transcriptional mechanisms reflect a cassette of genes and proteins that work collectively at the cellular, local, and systemic levels to compensate for a discrepancy in oxygen supply and demand. The findings suggest that under conditions where adaptive homeostatic mechanisms are appropriately engaged, damage to the brain can be substantially lessened or even prevented (Fig. 3). Stroke is, almost by definition, a failure of homeostasis. Accordingly, identification of small molecules that augment endogenous adaptive strategies provides a mechanism to tilt the balance away from cell damage and death and toward cell survival and repair (Fig. 3).
Fig. 3.
Drugs that augment endogenous homeostatic mechanisms will more effectively neutralize the heterogeneity inherent in stroke pathophysiology. As these pathways are already used by the body, their activation can occur with decreased threat of toxicity. The term “homeostasis” was coined by Walter Canon in the early twentieth century. It refers to the innate tendency of organisms to mobilize adaptive responses physiological and pathological perturbations that ultimately return the system to a set point that is consistent with survival. a The experimental paradigm of “ischemic preconditioning” has shown that a short, sublethal exposure to hypoxia, or hypoxia-ischemia induces homeostatic responses that make the organism “immune” or “tolerant” to a lethal ischemic insult. Mechanistic studies have revealed that tolerance is the consequence of activation of pre-existing proteins and de novo gene expression. b According to this model, stroke can be conceptualized as a failure of homeostasis. Consequently, neurons die and the brain is permanently damaged. c By extension, small molecules that engage homeostatic mechanisms designed to alleviate hypoxia/ischemia early or enhance their activation should tip the balance away from cell death and toward survival and repair. Such small molecules are currently being developed and represent a new generation of stroke therapies
Examination of adaptive responses to hypoxia in the central nervous system has affirmed that the family of transcriptional regulators known as the hypoxia-inducible factors are central players [25–27]. HIF-1 was purified and cloned as a result of a search for proteins that regulate the expression of genes involved in hypoxic adaptation, such as erythropoietin, vascular endothelial growth factor, and glycolytic enzymes [27]. HIF is a heterodimeric transcriptional activator composed of an inducible HIF-1α subunit and a constitutively expressed HIF-1β subunit [28, 29]. HIF-1α stability is regulated via the activity of a class of oxygen, 2-oxoglutarate, and iron dependent enzymes known as the HIF prolyl-4 hydroxylases (HIF PHDs, Fig. 4) [30]. As intracellular oxygen levels drop below a critical threshold, these enzymes fail to hydroxylate HIF-1α. As hydroxylation is required for the recruitment of the constitutively active E3 Ubiquitin Ligase, Von Hippel Lindau protein, HIF-1α becomes stabilized. HIF-1α can partner with its constitutively expressed but induced partner HIF-1β and translocate to the nucleus to regulate the expression of a host of genes involved in hypoxic adaptation [31]. Consistent with this model, several groups have shown that HIF-1α immunoreactivity increases in areas of the cortex that become hypoxic due to stroke [25, 32] An unanswered question has been whether HIF is also upregulated in areas connected to but remote from the site of ischemia. Indeed, neurons projecting to an area of infarction are at risk for cell death due to a loss of trophic support from their damaged targets. Moreover, neurons projecting from an area of damage are at risk for cell death due to a loss of trophic excitatory input from their targets. An elegant recent study in non-human primates demonstrated a dramatic increase in neuronal immunoreactivity for HIF-1α and one of its target genes in the infarct and peri-infarct region [33, 34]. Indeed, the neuronal immunoreactivity for HIF-1α increased from less than 5% to nearly 90% in both regions. Interestingly, areas remote to the area of hypoxia and ischemia also experience increased HIF and vascular endothelial growth factor (VEGF) levels but to a quantitatively much smaller extent. Together, the published rodent and primate histochemical studies support the hypothesis that increased HIF protein levels resulting from direct hypoxia and non-hypoxic mediators such as IGF-1 are a marker for surviving and regenerating neurons after ischemia [32].
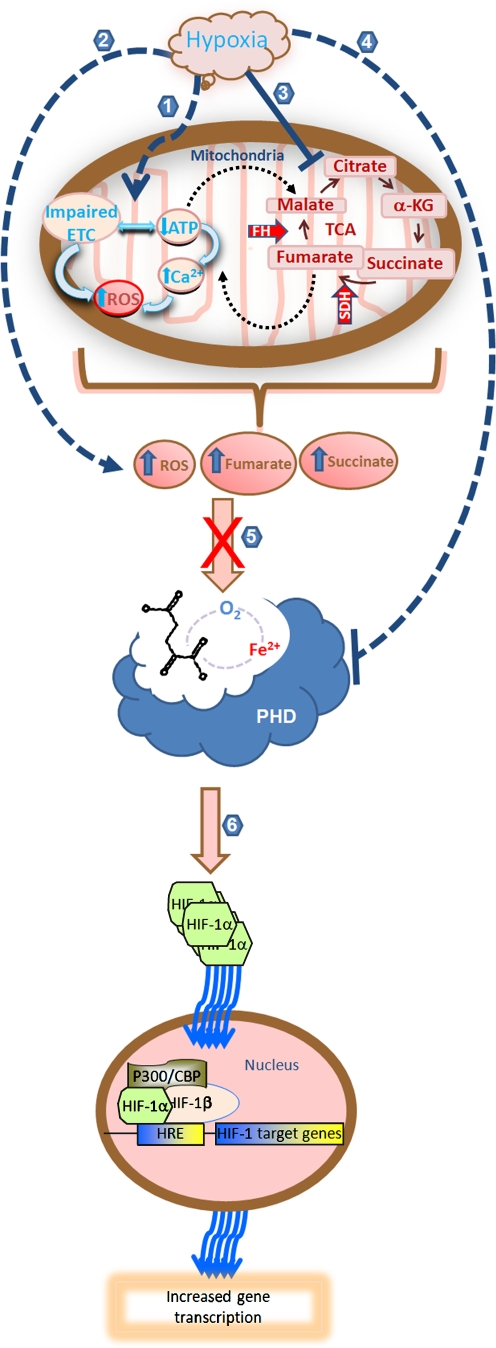
Fig. 4.
HIF prolyl 4 hydroxylases sense hypoxia and transduce a critical insufficiency in oxygen in the brain into transcriptional and post-transcriptional signal changes that mediate protection and repair. Hypoxia regulates the activity of HIF PHDs via direct or indirect mechanisms; production of peroxide via reduction in mitochondrial ATP production and electron transport chain (ETC) impairment (1, 2); accumulation of the tricarboxylic cycle (TCA) intermediates succinate and fumarate (3); or direct inhibition of the activity of PHDs due to lack of oxygen (4). Accumulation of hydrogen peroxide, succinate or fumarate can inhibits the activity of PHDs by competing with 2-oxoglutarate or by oxidizing the active site iron (5). Among its numerous downstream effects, inhibition of HIF PHD activity leads to stabilization of HIF-1α. Stabilized HIF-1α dimerizes with HIF-1β in the nucleus and increases gene transcription (6)
Pharmacological and molecular studies have provided additional support for the notion that stabilization of HIF-1 after ischemia is associated with enhanced survival of neurons. Small molecule hypoxia mimics, deferoxamine, and cobalt chloride, were found to stabilize HIF-1α levels, increase DNA binding to a cognate hypoxia response element, and increase the expression of HIF target genes in vitro cultured neurons (Zaman et al. [31]) and in vivo in the intact brain (Fig. 4; [35]). Pretreatment or post-treatment with desferrioxamine or cobalt chloride resulted in reduced cell loss in models of focal or global ischemia in vitro and in vivo [25, 31, 36–39]. Subsequent studies have confirmed that hypoxia, iron chelation, or cobalt chloride appear to confer protective effects on neurons via their ability to inhibit HIF PHDs [35]. Emerging data indicate that inhibition of each of the three of the HIF PHD isoforms (1–3) may lead to cell survival in the nervous system. Of note, inhibition of each isoform may enhance survival via distinct but mutually supportive pathways.
Our studies demonstrate that pharmacological inhibition of PHDs, in vitro, leads to inhibition of oxidative stress-induced death, an established mediator of neural injury and death in stroke [35]. More recent studies indicate that molecular suppression of HIF PHD 1 alone can mimic this effect (Siddiq et al., unpublished observations). Interestingly, while HIF-1, HIF-2, and cAMP response element-binding protein are stabilized by PHD inhibition in neurons or astrocytes, the molecular deletion or inhibition of each of these three transcription factors fails to abrogate the protective effects of PHD inhibition suggesting that other, as yet unidentified, pathways are important in protection (Siddiq et al., unpublished observations). Studies are underway to clarify whether molecular deletion of PHD1 selectively in the CNS confers resistance to stroke in a HIF-independent manner.
In contrast to PHD1, PHD2 appears to be the isoform most important for tagging HIF for degradation. Deletion of PHD2 but not PHD1 or PHD3 results in increased HIF and VEGF protein and consequent angiogenesis in multiple organs including the brain [40]. Underscoring PHD2’s important role in HIF signaling, the expression level of this isoform is significantly higher as compared to its brethren [41]. Ischemia-induced PHD2 inhibition stabilizes HIF and enhances expression of genes that mediate cellular (e.g., glycolytic enzymes), local (vascular endothelial growth factor), and systemic (erythropoietin, Epo) adaptive responses to hypoxia or hypoxia-ischemia [42]. While Epo is best known as a hematopoietic growth factor that can enhance oxygen carrying capacity to tissue, it also has organ-autonomous roles in the nervous system. Epo is produced in astrocytes in response to hypoxia or ischemia and mediates a number of responses critical to stroke prevention and recovery [43, 44]. Epo can inhibit neuronal death due to excitotoxicity or growth factor deprivation [45–49]. It can also stimulate the proliferation of neural progenitors in the germinal zones of the brain and enhance their migration to sites of injury [50, 51]. The concomitant HIF-dependent increase in VEGF expression provides, among other things, the appropriate angiogenic niche for neural progenitors to survive [52]. New neurons could mediate recovery responses via paracrine effects or due to direct participation in functional circuits. A recent study affirmed the requisite role for HIF transcription factors in mediating some of the salutary effects of low molecular weight PHD inhibitors given after cerebral ischemia [53]. Available evidence suggests that these inhibitors are likely targeting PHD2 to induce HIF and its gene targets in the CNS.
Inhibition of the third isoform of PHDs (PHD3) by hypoxia or hypoxia mimetics has also been linked to neuronal survival [54]. Freeman and colleagues first identified PHD3 (then known as SM-20) as a message and protein that is highly upregulated in sympathetic neurons after growth factor deprivation. Subsequent studies have shown that pharmacological or molecular deletion of HIF PHD3 prevents apoptosis associated with trophic loss in neurons and that the protection is HIF independent [55]. As target (post-synaptic) derived trophic factors appear to be lost after stroke and result in cell death remote from the infarct site, HIF PHD3 inhibition may be a rational strategy for maintaining the viability of these neurons in evolving or stable stroke.
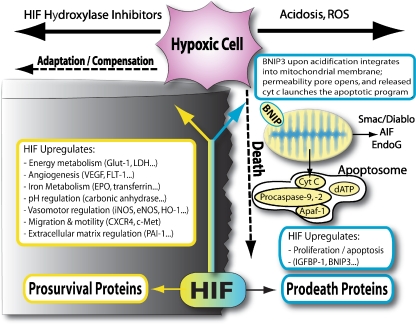
From the above discussion, a model begins to emerge whereby inhibition of HIF PHD1, 2, or 3 by hypoxia or consequences of hypoxia can mediate HIF-dependent and HIF-independent compensatory responses via distinct but clearly reinforcing mechanisms. How does one reconcile this model with observations from several laboratories, including our own, that HIF PHD inhibition or HIF activation leads to the upregulation of prodeath, Bcl-2 family proteins such as Puma, Bnip3, and NIX [56]? Moreover, constitutive HIF activation has been associated with potentiation and suppression of death [56]. These findings appear to make perfect sense if one considers apoptosis as an adaptive response to stress. After stroke, oxygen may fall below a critical level rendering the prolyl hydroxylases inactive. Accordingly, HIF is stabilized and it translocates to the nucleus to upregulate genes involved in preventing and executing death. The anthropomorphized cell can be visualized stepping to the edge of a steep cliff in response to a potentially lethal stress (Fig. 5). If adequate compensation for hypoxia occurs, prodeath proteins are not activated and the cell survives as a functional component of a complex neural network—the cell moves away from the cliff to survival promoting, terra firma. By contrast, if oxidative stress, hypoxia, and acidosis dominate, indicating that compensatory mechanisms have failed, then proapoptotic proteins such as BNIP3 undergo a conformational change, insertion into the mitochondrial membrane and activation of permeabilization transition and release of apoptotic effectors [57–59]. In the latter scenario, the cell jumps off the cliff to preserve limiting resources for its neighbors. The model suggests that state changes in the cell (e.g., redox and pH) that follow the initial ischemic insult will determine the fate of the tissue and potential for recovery (Fig. 5). Of note, low molecular weight or peptide inhibitors of the PHDs, antioxidants, and a small interfering RNA to BNIP3 prevent the prodeath effects of HIF (Amino et al., personal communication). These findings suggest that engaging the adaptive response at an upstream point where stress is detected by the cell (the stress sensor) is going to be more effective in stemming cell loss and facilitating repair than at the point of the transcription factor (HIF). It is our hypothesis that engaging the adaptive response at an upstream point in the pathway results in post-transcriptional changes essential for the homeostatic response. These changes in concert with transcriptional changes optimally alleviate the discrepancy between oxygen supply and demand.
Fig. 5.
Adaptation to hypoxia-cell fate and beyond. Expression of HIF in neurons leads to the constitutive expression of proteins associated with cell death (BNIP3, NIX, and PUMA) and cell survival (VEGF, glycolytic enzymes, Epo, and p21 waf1/cip1). Similar prodeath gene expression is found in neurons exposed to hypoxia or hypoxia mimetics despite the absence of cell death. It appears the oxygen “starved” neurons have stepped to the edge of the cliff. If during the ensuing hours to days the neuron becomes acidotic or oxidized, then prodeath proteins such as BNIP3 undergo a conformational change, insertion into the mitochondrial membrane, release of apoptotic effectors, and death. By contrast, if the survival genes are effective in neutralizing the hypoxic stress (e.g., no acidosis or oxidative stress), then the death genes never get activated. Our studies indicate that antioxidants, short interfering RNAs to BNIP3 or inhibitors of the HIF prolyl 4 hydroxylases tip the balance toward survival (away from the cliff)
The model has some clear predictions. First, low molecular weight global inhibitors of the HIF prolyl 4 hydroxylases will be more effective at preventing injury and repairing damage after stroke than selective isoform inhibitors. These inhibitors will engage HIF-dependent and HIF-independent pathways at cellular, local, and systemic levels and ultimately alleviate the discrepancy in nutrient supply and demand. They can also (via mechanisms that are only beginning to be defined) divert HIF away from its tendencies as a prodeath transcription factor. The ability of single “drugs” to target an oligopoly of proteins (HIF PHD1–3) to affect a concerted program of neuroprotection involving more than 70 genes and larger number of proteins suggests a strategy for overcoming the heterogeneity inherent in stroke pathophysiology in the short term. While a significant amount of work needs to be done to adequately assess the viability of this strategy for human therapeutics, the notion of augmenting endogenous adaptive programs via HIF PHDs to thwart disease continues to gain currency.
Acknowledgements
The authors wish to thank Gregg Semenza for reagents and intellectual input on all of the studies related to hypoxia in our laboratory. This work was supported by a New York State Center of Research Excellence, NIH RO1 (NS 40591, NS46239, and NS37060) and the Adelson Foundation for Neurorehabilitation and Repair.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Savitz SI, Fisher M (2007) Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann Neurol 61:396–402 [DOI] [PubMed]
- 2.Sharbrough FW, Messick JM Jr., Sundt TM Jr. (1973) Correlation of continuous electroencephalograms with cerebral blood flow measurements during carotid endarterectomy. Stroke 4:674–683 [DOI] [PubMed]
- 3.Naritomi H, Sasaki M, Kanashiro M, Kitani M, Sawada T (1988) Flow thresholds for cerebral energy disturbance and Na+ pump failure as studied by in vivo 31P and 23Na nuclear magnetic resonance spectroscopy. J Cereb Blood Flow Metab 8:16–23 [DOI] [PubMed]
- 4.Gibson GE, Pulsinelli W, Blass JP, Duffy TE (1981) Brain dysfunction in mild to moderate hypoxia. Am J Med 70:1247–1254 [DOI] [PubMed]
- 5.Hossmann KA, Ophoff BG, Csiba L, Paschen W (1988) Regional pH and electrolyte homeostasis of cat brain after prolonged ischemia. Neurochem Pathol 9:127–137 [DOI] [PubMed]
- 6.Astrup J, Nordstrom CH, Rehncrona S (1977) Rate of rise in extracellular potassium in the ischemic rat brain and the effect of preischemic metabolic rate: evidence for a specific effect of Phenobarbital. Acta Neurol Scand Suppl 64:148–149 [PubMed]
- 7.Harris RJ, Symon L (1984) Extracellular pH, potassium, and calcium activities in progressive ischaemia of rat cortex. J Cereb Blood Flow Metab 4:178–186 [DOI] [PubMed]
- 8.Choi DW (1988) Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci 11:465–469 [DOI] [PubMed]
- 9.Iadecola C, Yang G, Ebner TJ, Chen G (1997) Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol 78:651–659 [DOI] [PubMed]
- 10.Siesjo BK (1988) Historical overview. Calcium, ischemia, and death of brain cells. Ann NY Acad Sci 522:638–661 [DOI] [PubMed]
- 11.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH (1991) Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A 88:6368–6371 [DOI] [PMC free article] [PubMed]
- 12.Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424 [DOI] [PMC free article] [PubMed]
- 13.Yu SW, Wang H, Poitras MF et al (2002) Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297:259–263 [DOI] [PubMed]
- 14.Aarts MM, Tymianski M (2005) TRPMs and neuronal cell death. Pflugers Arch 451:243–249 [DOI] [PubMed]
- 15.Xiong ZG, Chu XP, Simon RP (2007) Acid sensing ion channels—novel therapeutic targets for ischemic brain injury. Front Biosci 12:1376–1386 [DOI] [PubMed]
- 16.Lyden P, Wahlgren NG (2000) Mechanisms of action of neuroprotectants in stroke. J Stroke Cerebrovasc Dis 9:9–14 [DOI] [PubMed]
- 17.Ito N, DeMarco RA, Mailliard RB et al (2007) Cytolytic cells induce HMGB1 release from melanoma cell lines. J Leukoc Biol 81:75–83 [DOI] [PubMed]
- 18.MacManus JP, Buchan AM (2000) Apoptosis after experimental stroke: fact or fashion? J Neurotrauma 17:899–914 [DOI] [PubMed]
- 19.Goldberg JL (2003) How does an axon grow? Genes Dev 17:941–958 [DOI] [PubMed]
- 20.Coleman MP, Perry VH (2002) Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci 25:532–537 [DOI] [PubMed]
- 21.Fisher M, Ratan R (2003) New perspectives on developing acute stroke therapy. Ann Neurol 53:10–20 [DOI] [PubMed]
- 22.Cho S, Park EM, Zhou P, Frys K, Ross ME, Iadecola C (2005) Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J Cereb Blood Flow Metab 25:493–501 [DOI] [PubMed]
- 23.Stenzel-Poore MP, Stevens SL, Xiong Z et al (2003) Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet 362:1028–1037 [DOI] [PubMed]
- 24.Tang Y, Pacary E, Freret T et al (2006) Effect of hypoxic preconditioning on brain genomic response before and following ischemia in the adult mouse: identification of potential neuroprotective candidates for stroke. Neurobiol Dis 21:18–28 [DOI] [PubMed]
- 25.Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR (2000) Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol 48:285–296 [DOI] [PubMed]
- 26.Semenza GL (2000) Chairman’s summary: mechanisms of oxygen homeostasis, circa 1999. Adv Exp Med Biol 475:303–310 [DOI] [PubMed]
- 27.Wang GL, Semenza GL (1993) Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem 268:21513–21518 [PubMed]
- 28.Wang GL, Jiang BH, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92:5510–5514 [DOI] [PMC free article] [PubMed]
- 29.Wang GL, Semenza GL (1995) Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 270:1230–1237 [DOI] [PubMed]
- 30.Kaelin WG (2005) The von Hippel-Lindau tumor suppressor protein: roles in cancer and oxygen sensing. Cold Spring Harb Symp Quant Biol 70:159–166 [DOI] [PubMed]
- 31.Zaman K, Ryu H, Hall D et al (1999) Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci 19:9821–9830 [DOI] [PMC free article] [PubMed]
- 32.Chavez JC, LaManna JC (2002) Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci 22:8922–8931 [DOI] [PMC free article] [PubMed]
- 33.Stowe AM, Plautz EJ, Nguyen P, et al. (2007) Neuronal HIF-1alpha protein and VEGFR-2 immunoreactivity in functionally related motor areas following a focal M1 infarct. J Cereb Blood Flow Metab (in press). DOI 10.1038/sj.jcbfm.9600560 [DOI] [PMC free article] [PubMed]
- 34.Stowe AM, Plautz EJ, Eisner-Janowicz I et al (2007) VEGF protein associates to neurons in remote regions following cortical infarct. J Cereb Blood Flow Metab 27:76–85 [DOI] [PMC free article] [PubMed]
- 35.Siddiq A, Ayoub IA, Chavez JC et al (2005) Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem 280:41732–41743 [DOI] [PMC free article] [PubMed]
- 36.Demougeot C, Van Hoecke M, Bertrand N et al (2004) Cytoprotective efficacy and mechanisms of the liposoluble iron chelator 2,2′-dipyridyl in the rat photothrombotic ischemic stroke model. J Pharmacol Exp Ther 311:1080–1087 [DOI] [PubMed]
- 37.Demougeot C, Bobillier-Chaumont S, Mossiat C, Marie C, Berthelot A (2004) Effect of diets with different magnesium content in ischemic stroke rats. Neurosci Lett 362:17–20 [DOI] [PubMed]
- 38.Hamrick SE, McQuillen PS, Jiang X, Mu D, Madan A, Ferriero DM (2005) A role for hypoxia-inducible factor-1alpha in desferoxamine neuroprotection. Neurosci Lett 379:96–100 [DOI] [PubMed]
- 39.Sorond FA, Ratan RR (2000) Ironing-out mechanisms of neuronal injury under hypoxic-ischemic conditions and potential role of iron chelators as neuroprotective agents. Antioxid Redox Signal 2:421–436 [DOI] [PubMed]
- 40.Takeda K, Fong GH (2007) Prolyl hydroxylase domain 2 protein suppresses hypoxia-induced endothelial cell proliferation. Hypertension 49:178–184 [DOI] [PubMed]
- 41.Appelhoff RJ, Tian YM, Raval RR et al (2004) Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279:38458–38465 [DOI] [PubMed]
- 42.Natarajan J, Berrar D, Dubitzky W et al (2006) Text mining of full-text journal articles combined with gene expression analysis reveals a relationship between sphingosine-1-phosphate and invasiveness of a glioblastoma cell line. BMC Bioinformatics 7:373 [DOI] [PMC free article] [PubMed]
- 43.Chavez JC, Baranova O, Lin J, Pichiule P (2006) The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci 26:9471–9481 [DOI] [PMC free article] [PubMed]
- 44.Yeo EJ, Cho YS, Kim MS, Park JW (2007) Contribution of HIF-1alpha or HIF-2alpha to erythropoietin expression: in vivo evidence based on chromatin immunoprecipitation. Ann Hematol 87:11–17 [DOI] [PubMed]
- 45.Cerami A, Brines ML, Ghezzi P, Cerami CJ (2001) Effects of epoetin alfa on the central nervous system. Semin Oncol 28:66–70 [DOI] [PubMed]
- 46.Ehrenreich H, Hasselblatt M, Dembowski C et al (2002) Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med 8:495–505 [PMC free article] [PubMed]
- 47.Liu R, Suzuki A, Guo Z, Mizuno Y, Urabe T (2006) Intrinsic and extrinsic erythropoietin enhances neuroprotection against ischemia and reperfusion injury in vitro. J Neurochem 96:1101–1110 [DOI] [PubMed]
- 48.Prass K, Scharff A, Ruscher K et al (2003) Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke 34:1981–1986 [DOI] [PubMed]
- 49.Wei L, Han BH, Li Y, Keogh CL, Holtzman DM, Yu SP (2006) Cell death mechanism and protective effect of erythropoietin after focal ischemia in the whisker-barrel cortex of neonatal rats. J Pharmacol Exp Ther 317:109–116 [DOI] [PubMed]
- 50.Shingo T, Sorokan ST, Shimazaki T, Weiss S (2001) Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci 21:9733–9743 [DOI] [PMC free article] [PubMed]
- 51.Tsai MS, Hwang SM, Tsai YL, Cheng FC, Lee JL, Chang YJ (2006) Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol Reprod 74:545–551 [DOI] [PubMed]
- 52.Goldman CK, Kendall RL, Cabrera G et al (1998) Paracrine expression of a native soluble vascular endothelial growth factor receptor inhibits tumor growth, metastasis, and mortality rate. Proc Natl Acad Sci U S A 95:8795–8800 [DOI] [PMC free article] [PubMed]
- 53.Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC (2007) Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci 27:6320–6332 [DOI] [PMC free article] [PubMed]
- 54.Lomb DJ, Straub JA, Freeman RS (2007) Prolyl hydroxylase inhibitors delay neuronal cell death caused by trophic factor deprivation. J Neurochem 103:1897–1906 [DOI] [PubMed]
- 55.Lee S, Nakamura E, Yang H et al (2005) Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell 8:155–167 [DOI] [PubMed]
- 56.Aminova LR, Chavez JC, Lee J et al (2005) Prosurvival and prodeath effects of hypoxia-inducible factor-1alpha stabilization in a murine hippocampal cell line. J Biol Chem 280:3996–4003 [DOI] [PubMed]
- 57.Bruick RK (2000) Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci U S A 97:9082–9087 [DOI] [PMC free article] [PubMed]
- 58.Frazier DP, Wilson A, Graham RM, Thompson JW, Bishopric NH, Webster KA (2006) Acidosis regulates the stability, hydrophobicity, and activity of the BH3-only protein Bnip3. Antioxid Redox Signal 8:1625–1634 [DOI] [PubMed]
- 59.Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA (2002) Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci U S A 99:12825–12830 [DOI] [PMC free article] [PubMed]