Abstract
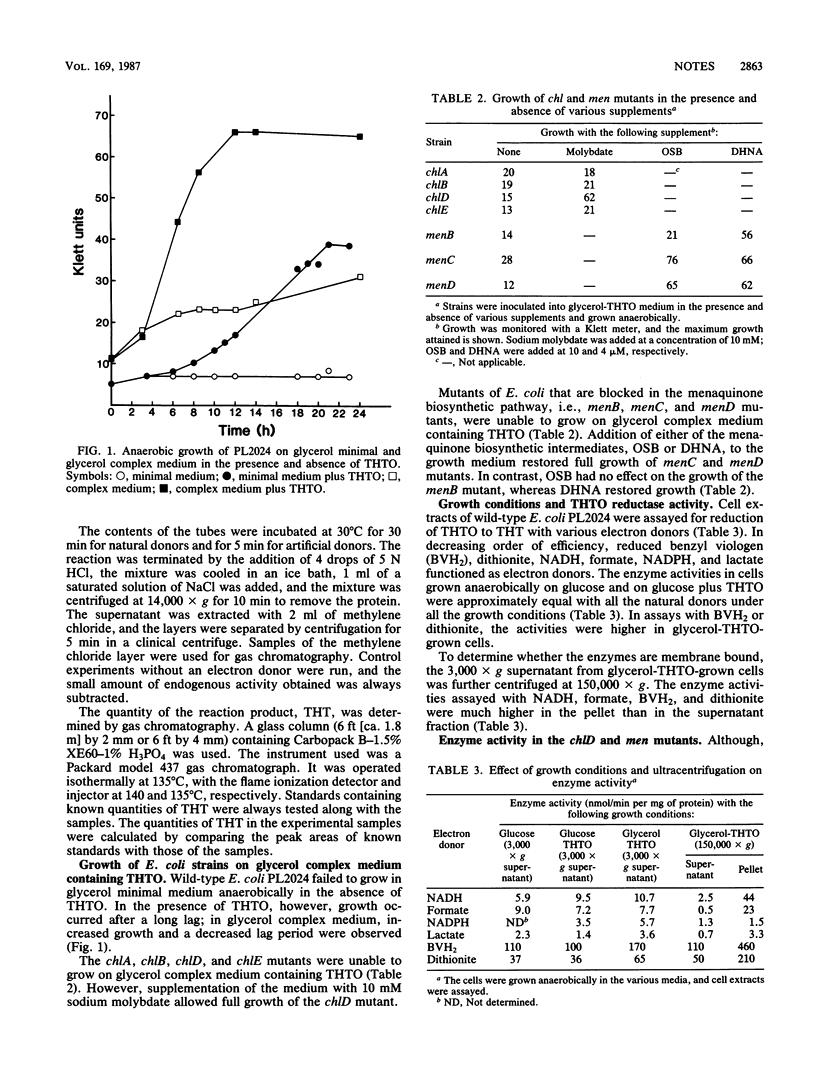
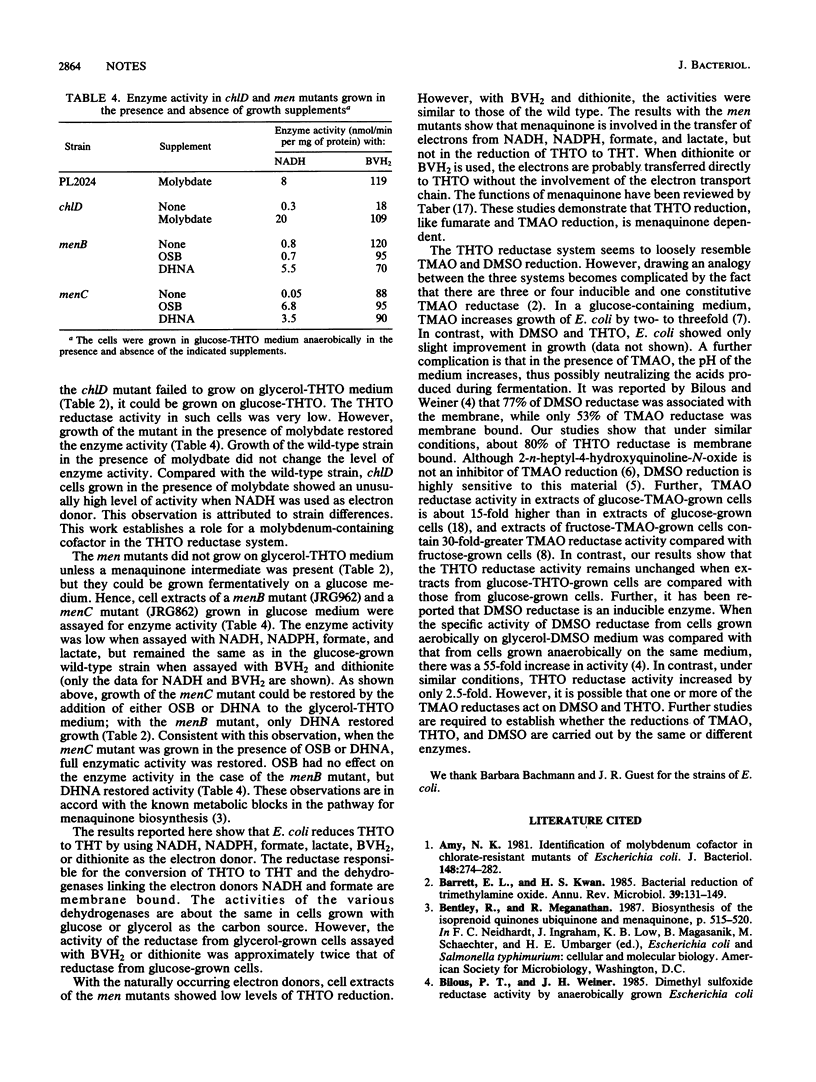
Escherichia coli used tetrahydrothiophene 1-oxide (THTO) as an electron acceptor for anaerobic growth with glycerol as a carbon source; the THTO was reduced to tetrahydrothiophene. Cell extracts also reduced THTO to tetrahydrothiophene in the presence of a variety of electron donors. Chlorate-resistant (chl) mutants (chlA, chlB, chlD, and chlE) were unable to grow with THTO as the electron acceptor. However, growth and THTO reduction by the chlD mutant were restored by high concentrations of molybdate. Similarly, mutants of E. coli that are blocked in the menaquinone (vitamin K2) biosynthetic pathway, i.e., menB, menC, and menD mutants, did not grow with THTO as an electron acceptor. Growth and THTO reduction were restored in these mutants by the presence of appropriate intermediates of the vitamin K biosynthetic pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy N. K. Identification of the molybdenum cofactor in chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1981 Oct;148(1):274–282. doi: 10.1128/jb.148.1.274-282.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. L., Kwan H. S. Bacterial reduction of trimethylamine oxide. Annu Rev Microbiol. 1985;39:131–149. doi: 10.1146/annurev.mi.39.100185.001023. [DOI] [PubMed] [Google Scholar]
- Bilous P. T., Weiner J. H. Dimethyl sulfoxide reductase activity by anaerobically grown Escherichia coli HB101. J Bacteriol. 1985 Jun;162(3):1151–1155. doi: 10.1128/jb.162.3.1151-1155.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilous P. T., Weiner J. H. Proton translocation coupled to dimethyl sulfoxide reduction in anaerobically grown Escherichia coli HB101. J Bacteriol. 1985 Jul;163(1):369–375. doi: 10.1128/jb.163.1.369-375.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Hackett N. R. Cytochromes of the trimethylamine N-oxide anaerobic respiratory pathway of Escherichia coli. Biochim Biophys Acta. 1983 Oct 31;725(1):168–177. doi: 10.1016/0005-2728(83)90237-2. [DOI] [PubMed] [Google Scholar]
- Cox J. C., Madigan M. T., Favinger J. L., Gest H. Redox mechanisms in "oxidant-dependent" hexose fermentation by Rhodopseudomonas capsulata. Arch Biochem Biophys. 1980 Oct 1;204(1):10–17. doi: 10.1016/0003-9861(80)90002-8. [DOI] [PubMed] [Google Scholar]
- Guest J. R. Anaerobic growth of Escherichia coli K12 with fumarate as terminal electron acceptor. Genetic studies with menaquinone and fluoroacetate-resistant mutants. J Gen Microbiol. 1979 Dec;115(2):259–271. doi: 10.1099/00221287-115-2-259. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Poole R. K. The respiratory chains of Escherichia coli. Microbiol Rev. 1984 Sep;48(3):222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambden P. R., Guest J. R. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J Gen Microbiol. 1976 Dec;97(2):145–160. doi: 10.1099/00221287-97-2-145. [DOI] [PubMed] [Google Scholar]
- McGovern E. P., Bentley R. Isolation and properties of naphthoate synthetase from Mycobacterium phlei. Arch Biochem Biophys. 1978 May;188(1):56–63. doi: 10.1016/0003-9861(78)90355-7. [DOI] [PubMed] [Google Scholar]
- Shaw D. J., Guest J. R., Meganathan R., Bentley R. Characterization of Escherichia coli men mutants defective in conversion of o-succinylbenzoate to 1,4-dihydroxy-2-naphthoate. J Bacteriol. 1982 Dec;152(3):1132–1137. doi: 10.1128/jb.152.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Brock T. D. Dimethyl sulfoxide as an electron acceptor for anaerobic growth. Arch Microbiol. 1978 Jan 23;116(1):35–40. doi: 10.1007/BF00408731. [DOI] [PubMed] [Google Scholar]
- Zinder S. H., Brock T. D. Dimethyl sulphoxide reduction by micro-organisms. J Gen Microbiol. 1978 Apr;105(2):335–342. doi: 10.1099/00221287-105-2-335. [DOI] [PubMed] [Google Scholar]


