Abstract
The murine yolk sac, being the first site of embryonic blood cell production, has long been theorized to contain the migrating hematopoietic stem cells (HSC) that seed the liver and initiate hematopoiesis on day 10.0 postcoitus (pc). However, it remains controversial whether yolk sac cells isolated before day 11.0 pc possess any long-term repopulating HSC activity upon transplantation into adult recipient mice. We hypothesized that failure to demonstrate engraftment of day <11.0 yolk sac cells in adult hosts may result from an inability of yolk sac cells to home to the active adult hematopoietic sites (spleen and bone marrow). In the present studies, we transplanted yolk sac cells into conditioned newborn mice in whom the liver, as well as the spleen and bone marrow, concomitantly function as a site of blood cell formation. We report that yolk sac cells isolated from day 9.0 pc embryos provide long-term multilineage reconstitution for at least 11 months in primary conditioned newborn mice and for at least 6 months in secondary recipients. Donor yolk sac HSC progeny repopulated mature peripheral blood, thymus, spleen, and bone marrow lymphoid, myeloid, and erythroid compartments. Thus, day 9.0 pc yolk sac HSC can contribute to definitive multilineage hematopoiesis in transplanted recipients. Determination of HSC activity in the day 9.0 pc murine yolk sac suggests that yolk sac HSC are available to seed the liver on day 10.0 pc when definitive hematopoiesis is initiated.
Keywords: transplantation, ontogeny, cell differentiation
In the mouse embryo, blood cells first appear at day 7.5 postcoitus (pc), when primitive erythrocytes are detectable in yolk sac blood islands (1). The liver primordium develops on day 9.0 (2) and 24 h later is colonized with hematopoietic cells (3). By day 12.0 pc, the liver is the predominant site of blood cell formation in the embryo. For many years, investigators have questioned whether the yolk sac blood islands contain hematopoietic stem cells (HSC) that not only differentiate into the primitive erythrocytes, but also migrate to the developing liver to initiate definitive hematopoiesis (4). In the developing chick, orthotopic-grafting experiments have revealed that HSC contributing to definitive chicken hematopoiesis are derived from intraembryonic tissue (containing the dorsal aorta) while the extraembryonic yolk sac only contributes to transient primitive hematopoiesis (5–7). Investigators have been unable to perform similar cell fate or orthotopic-grafting experiments in utero in the mouse and, therefore, have relied on comparative in vivo and in vitro hematopoietic assays of microdissected embryonic tissues to define the characteristics of the earliest embryonic HSC (8, 9).
Murine yolk sac cells from day ≤11.0 pc embryos give rise to multilineage repopulating cells upon in utero transplantation into murine embryos (10) and individual lineages of mature T lymphocytes (10, 11), B lymphocytes (12, 13), phagocytes (1), or erythroid cells (14) when transplanted into lethally irradiated or immune deficient adult recipient mice. Multipotent hematopoietic progenitor cells (15) isolated from day ≤11.0 pc yolk sacs have been cultured in vitro and yolk sac cells have been demonstrated to colonize fetal liver rudiments in vitro (16). Coculture of day 10 pc yolk sac hematopoietic cells with cloned yolk sac endothelial cell lines permits recovery of multipotent progenitor and short-term in vivo multilineage repopulating cells several weeks later (17). However, intraembryonic sites of murine hematopoiesis, with demonstrable HSC (18), colony forming units-spleen (19), and multipotent progenitor cell activity (15, 20), have also been identified in day 8.0–10.0 pc murine embryos. Recently, HSC with demonstrable long-term repopulating activity in lethally irradiated adult recipients were identified in the aorta-gonad-mesonephros (AGM) region of the embryo on day 10.0 pc, prior to such activity being observed in the yolk sac or fetal liver (21).
We and others (17, 21, 22) have postulated that the inability to demonstrate long-term multilineage repopulating HSC in the day <11.0 pc embryonic yolk sac could be secondary to a failure of the embryonic cells to find appropriate homing sites within the hematopoietic microenvironments of lethally irradiated adult recipient mice. Because hematopoiesis in the newborn mouse is known to occur simultaneously in the liver, spleen, and bone marrow (23) and because the sequential sites of blood cell production occur in the order of the yolk sac, liver, spleen, and then bone marrow during murine ontogeny (24, 25), we hypothesized that yolk sac cells would engraft in newborn animals with robust liver hematopoiesis, but not in adult recipients in whom the liver is no longer hematopoietically active.
In the present experiments, we isolated yolk sac cells from 13–20 somite staged (day 9.0 pc) embryos and transplanted the donor cells into newborn congenic mice that were conditioned in utero via busulfan administration to pregnant dams (26). We report that HSC are present in the day 9.0 pc yolk sac. The yolk sac HSC provided long-term multilineage reconstitution for at least 11 months in the primary recipients and for at least 6 months in secondary recipients. Donor yolk sac HSC progeny repopulated mature peripheral blood, thymus, spleen, and bone marrow lymphoid, myeloid, and erythroid compartments in a pattern and frequency indistinguishable from normal age-matched animals. These results indicate that day 9.0 pc yolk sac HSC are capable of contributing to definitive hematopoiesis in vivo and that HSC are present in the murine yolk sac before the liver is seeded by migrating HSC on day 10.0 pc.
MATERIALS AND METHODS
Animal Care and Yolk Sac Isolation.
A C57BL/6J breeding colony was used to generate timed pregnant females from whom somite staged donor yolk sac cells were derived. A second breeding colony of B6. hemoglobin diffuse (Hbbd/Hbbd), glucose phosphate isomerase-1a (Gpi-1a/Gpi-1a) animals (B6.Hbbd,Gpi-1a) served to generate the newborn recipient mice. These animals are congenic with the C57BL/6J donors who are Hbbs/Hbbs (hemoglobin single) and Gpi-1b/Gpi-1b. Female mice were exposed to males from 0600–0900, and observation of the vaginal plug was designated as day 0 of gestation. All animals were cared for in our Laboratory Animal Resource Center by supervised trained technicians, and these studies were approved by our Institutional Animal Care and Use Committee.
Yolk sac cells were obtained as described (27). Briefly, timed-mated C57BL/6J females were killed by cervical dislocation. The uterine horns were removed, washed extensively in Hanks’ balanced salt solution (HBSS), and the day 9.0 pc embryos (13–20 somites) were harvested. The yolk sacs were dissected free and drawn through a 23G needle before transferring to a 10-cm dish (Corning) and incubated with 0.1% collagenase (Sigma) in 20% fetal calf serum and HBSS for 60 min at 37°C. Following the mild digestion, dispersed yolk sac cells were drawn through the 23G needle into a syringe, deposited into a polystyrene tube, and pelleted at 500 × g for 10 min. The single cell suspension of yolk sac cells were counted, and viability was tested via Trypan blue exclusion criteria.
Transplantation Assay.
Busulfan (20 mg/ml in 1% carboxymethylcellulose; Sigma) was injected subcutaneously at a dose of 15 mg/kg to pregnant B6.Hbbd,Gpi-1a dams on day 18 pc to sublethally condition the newborn pups for high-level donor cell engraftment (26). The C57BL/6J yolk sac cells (1–10 embryo equivalents; 1 day 9.0 pc equivalent = 3.4 ± 0.8 × 104 yolk sac cells) were suspended in 25 μl HBSS and drawn into a 100 μl sterile glass syringe adapted with an injection guide (Hamilton). The cell suspension was directly injected into the fetal liver or into the facial vein of the conditioned newborn recipient animals. Peripheral blood of all transplanted recipient animals was sampled from 1 to 11 months posttransplant for assay of donor type Gpi-1 isoenzyme chimerism. In control experiments, 3 or 10 embryo equivalent doses of day 9.0 pc yolk sac cells were injected into the liver or the facial vein of 6–9 unconditioned newborn mice and the recipients were analyzed for donor cell chimerism as above (experiment were repeated twice). In addition, 25 μl of whole blood from sacrificed pregnant C57BL/6J dams was injected directly into the liver of 6 conditioned newborn recipient pups as a control for possible maternal blood contamination of the yolk sac preparations. Finally, a 10-embryo equivalent dose of yolk sac cells was injected intravenously into 3 lethally irradiated [11 Gy γ-irradiation administered at 96 cGy/min in two doses divided by 4 h using a 137Cs irradiator (Nordion, Kanata, Canada)] or 3 busulfan (50 mg/kg)-conditioned adult B6.Hbbd,Gpi-1a mice on three occasions to test for donor yolk sac cell engraftment.
When primary recipient animals (transplanted with a 3-embryo equivalent dose of yolk sac cells) had reached 6 months of age, 6 randomly selected animals (from pool of 10) were killed and bone marrow cells were isolated as described (28). Low-density (<1.077 g/cm3) bone marrow mononuclear cells were recovered from each animal and 3 × 106 cells from each primary recipient were intravenously injected into 3 secondary recipient B6.Hbbd,Gpi-1a adult animals that had been conditioned with total body irradiation. Peripheral blood of secondary recipient animals was obtained at intervals from 1 to 6 months posttransplant and analyzed for the presence of donor type Gpi-1 isoenzyme. Peripheral blood was also sampled for peripheral white blood cell and lymphocyte counts and thymus, spleen, and bone marrow were assayed for lymphocyte subset analysis. Differences in peripheral white blood cell counts, percent Gpi-1 isoenzyme type, and lymphocyte subset percentages between primary or secondary transplant recipients and normal control animals were determined using the Student’s t test.
Isolation of Peripheral Blood Cells.
Peripheral blood was drawn from the tail vein of the primary and secondary recipient animals into three heparinized capillary tubes (Monoject, St. Louis). The peripheral whole blood was spun in a hematocrit tube centrifuge (IEC MB Centrifuge; International Equipment) and the white blood cell buffy coat was cut from the capillary tube, placed in a polypropylene tube, and washed with buffer (HBSS plus 0.5% BSA plus 5 mM EDTA). Mononuclear cells from peripheral blood were pelleted and aliquoted into three tubes where 0.5–1 μg of rat anti-mouse Gr-1 (granulocytes), B220 (B lymphocytes), or CD4/CD8 (T lymphocytes) monoclonal antibodies (PharMingen) were individually added for a 15-min incubation on ice. The individually stained cell populations were pelleted (500 × g for 10 min), washed with buffer, repelleted, and resuspended in 80 μl buffer. To each tube, 1 μl of goat anti-rat IgG magnetic microbeads (Miltenyi Biotec, Sunnyvale, CA) was added for a 15-min incubation on ice, and the purified cell populations were obtained using a magnetic separation device as directed by the manufacturer (Miltenyi Biotec) and as described (26). Isolated populations of cells were restained with fluorescein isothiocyanate (FITC) conjugates of the same antibody and analyzed for purity using a FACStarPLUS instrument (Becton Dickinson). If the purity was ≤90%, the samples were sorted to obtain highly pure (>98%) cell populations.
Lymphocyte Subset Analysis and Isolation.
Peripheral blood, spleen, thymus, and bone marrow low-density mononuclear cells were isolated from primary and secondary recipient animals 6 months posttransplant and were incubated with 1 μg/106 cells rat anti-mouse CD16/32 (PharMingen) for 10 min on ice to block nonspecific (Fc) binding of lineage-specific monoclonal antibodies. The cells were pelleted, resuspended, and incubated with rat phycoerythrin or fluorescein isothiocyanate-conjugated monoclonal antibodies (1 μg/106 cells) to murine markers including B220, IgG, IgM, CD43, CD4, CD8, and CD3 or isotype control antibodies (PharMingen). Cell analysis and sorting was accomplished using a FACStarPLUS instrument (Becton Dickinson). Dead cells were gated on the basis of forward and side scatter. Sorted cells were analyzed for donor type Gpi-1.
Gpi-1 Assay.
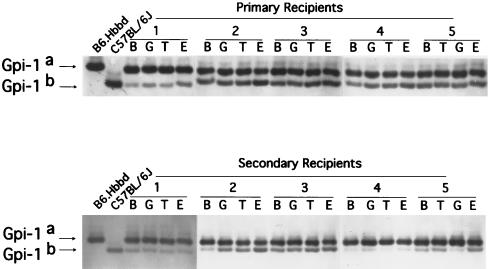
The isolated red blood cells, B and T lymphocytes, and granulocytes were deposited in separate microcentrifuge tubes and pelleted, and the cells were lysed by freeze-thawing in distilled water. To permit analysis of cell samples with low numbers of cells (10,000–50,000), we previously (26) determined that resuspending cells at 1,000 cells/μl of distilled water allowed recovery of sufficient enzyme for reproducible analysis (see Fig. 1). The cell lysates were applied in three applications to Titan III Zip Zone cellulose acetate plates (Helena Laboratories) using a Super Z 0.5 μl 8 well applicator (Helena Laboratories). The loaded gels were run for 30 min in an electrophoresis chamber at 300 V. The Gpi-1 isoenzymes were detected as recently described (26). Stained wet gels were immediately scanned with a Rep clinical densitometer (Helena Laboratories) for quantitation.
Figure 1.
Analysis of peripheral blood of randomly selected transplanted conditioned newborn primary and secondary recipient animals for evidence of day 9.0 pc yolk sac donor type Gpi-1 isoenzyme 4 months posttransplant. Donor yolk sac cells were derived from C57BL/6J animals expressing Gpi-1b and recipient congenic B6. Hbbd, Gpi-1a express Gpi-1a. While donor and recipient animals express multiple Gpi-1 isoenzymes, C57BL/6J animals express predominantly Gpi-1b (and no Gpi-1a) and B6. Hbbd,Gpi-1a express predominantly Gpi-1a (and no Gpi-1b). Analysis of percent donor derived cells in the primary and secondary recipients was performed by scanning only for Gpi-1b and Gpi-1a activity. Evidence of donor type Gpi-1b is present in B and T lymphocytes (B and T), granulocytes (G), and red blood cells (E) isolated from the blood of the five representative primary and five secondary recipient animals.
RESULTS
Donor Day 9.0 pc Yolk Sac Cells Engraft and Contribute to Long-Term Hematopoietic Reconstitution in Conditioned Newborn, But Not Adult, Recipients.
We were interested in determining if HSC activity was present in the murine yolk sac prior to the time that hematopoiesis is initiated in the developing liver (day 10.0 pc; 28 somite stage). Therefore, we administered day 9.0 pc single cell suspensions of yolk sac cells via direct liver injection or intravenously into <24-h-old congenic myeloablated newborn pups that had been conditioned in utero following intrapartum administration of busulfan to the pregnant dams. We observed that day 9.0 pc yolk sac cells engrafted and contributed mature blood cells to the peripheral blood of the sublethally conditioned newborn recipients for 11 months posttransplant (Tables 1 and 2). High level engraftment was present in recipients that received donor yolk sac cells by either intraliver or intravenous (Fig. 1) injection; however, the level of chimerism was significantly higher in the recipient transplanted with a 3-embryo equivalent dose via intraliver as compared with intravenous injection (Table 1, P < 0.05). The level of peripheral blood chimerism was also significantly higher in recipient animals transplanted with 10 as compared with 3-embryo equivalent doses of donor yolk sac cells (Table 1, P < 0.01). At 11 months posttransplant, engrafted donor day 9.0 pc yolk sac cells continued to contribute progeny to lymphoid, myeloid, and erythroid lineages of the peripheral blood of the primary recipient animals (Table 2). In contrast, no evidence of donor yolk sac cell chimerism was observed in any of the transplanted nonmyeloablated newborn pups at any time during a 6 month sequential analysis posttransplant (data not shown). Furthermore, whole blood of pregnant dams did not engraft and contribute to peripheral blood chimerism in any of the transplanted conditioned newborns (data not shown). Finally, day 9.0 pc yolk sac cells did not engraft and contribute to reconstitution of hematopoiesis in any (n = 18) lethally conditioned adult recipient animals transplanted (data not shown).
Table 1.
Percent donor Gpi-1 isoenzyme present in red and white blood cells of 11-month-old primary newborn recipients of day 9.0 pc yolk sac cells delivered via intraliver (IL) or intravenous (IV) injection
| Cell dose | % donor Gpi-1
|
|
|---|---|---|
| Red blood cells | White blood cells | |
| 1 embryo equivalent | ||
| IL | ND | ND |
| IV | 13 ± 4 | 9 ± 8 |
| 3 embryo equivalents | ||
| IL | 31 ± 5 | 34 ± 4 |
| IV | 20 ± 6 | 21 ± 7 |
| 10 embryo equivalents | ||
| IL | 52 ± 14 | 49 ± 10 |
| IV | ND | ND |
Mean ± SD values of 12–18 recipients. ND, not done. All 12 recipients of IV and all 18 recipients of IL injections were reconstituted.
Table 2.
Percent donor Gpi-1 isoenzyme in peripheral blood of primary recipients reconstituted with a 3-embryo equivalent dose of day 9.0 yolk sac cells and secondary hosts transplanted with bone marrow from the primary newborn recipients
| Cell source | % donor Gpi-1
|
|||
|---|---|---|---|---|
| B | T | G | R | |
| Primary recipients | 28 ± 7 | 31 ± 9 | 33 ± 5 | 35 ± 8 |
| Secondary recipients | 36 ± 6 | 36 ± 6 | 39 ± 5 | 40 ± 8 |
Mean ± SD values of B lymphocytes (B), T lymphocytes (T), granulocytes (G), or red blood cells (R) of 6 representative animals from 30 primary recipients (all reconstituted) at 11 months posttransplant and from 6 representative animals from 18 secondary recipients (all reconstituted) 6 months posttransplant.
Donor Day 9.0 pc Yolk Sac Cells Contribute to Long-Term Multilineage Hematopoietic Reconstitution in Secondary Recipient Animals.
Low-density bone marrow cells isolated 6 months posttransplant from primary recipients of donor yolk sac cells were administered via intravenous injection into lethally irradiated adult secondary recipients. Donor contributions to peripheral blood lymphoid, myeloid, and erythroid lineages were observed from 1 (ranging from 5 to 10% donor type Gpi-1 enzyme in each lineage) thru 6 months (Table 2) posttransplant (Fig. 1).
Donor Yolk Sac Cells Reconstitute Hematopoiesis in Primary and Secondary Recipients in a Pattern Indistinguishable from Normal Hematopoiesis.
Because hematopoiesis in the murine yolk sac at day 9.0 pc is restricted to the production of primitive erythrocytes and some macrophages (24), we examined the primary and secondary recipients to determine the distribution of mature blood cells in selected organs and in the peripheral blood. Peripheral blood hematocrits in the primary and secondary recipients were similar to normal age-matched animals 2 months posttransplant (43 ± 4%, 42 ± 3%, and 45 ± 5%, respectively) and at each time point examined thereafter. Peripheral white blood cell counts were also similar in the primary and secondary recipients compared with the normal animals at 2 months posttransplant (4660 ± 574 cells/μl, 5170 ± 950 cell/μl, and 4710 ± 917 cells/μl, respectively) and at each time thereafter. No differences in peripheral white blood cell differentials or red blood cell morphology were observed in these three study groups at any time examined from 2 months posttransplant or thereafter (data not shown). Isolation and Gpi-1 analysis of selected lymphocyte subsets in the spleen, bone marrow, and thymus of secondary recipients indicated that engrafted donor cells were capable of differentiating and producing progeny in all of the subsets tested (Table 3). Similar results were obtained when analyzing donor cell contributions to lymphocyte subsets in the hematopoietic organs of primary recipients.
Table 3.
Percent donor Gpi-1 isoenzyme in B and T cell subsets from 6 month old secondary recipient animals
| Secondary recipient lymphocyte subsets | % donor Gpi-1, mean ± SD |
|---|---|
| Thymus | |
| CD4+ CD8+ | 21 ± 3 |
| CD4− CD8− | 28 ± 9 |
| CD4+ | 22 ± 6 |
| CD8+ | 34 ± 8 |
| CD4+ CD3+ | 18 ± 11 |
| CD8+ CD3+ | 27 ± 5 |
| Bone marrow | |
| B220+ IgG+ | 33 ± 7 |
| B220+ IgD+ | 31 ± 12 |
| B220+ CD43+ | 26 ± 10 |
| B220+ | 21 ± 10 |
| CD43+ | 29 ± 12 |
| Spleen | |
| B220+ IgD+ | 24 ± 4 |
| B220+ IgM+ | 24 ± 8 |
| B220+ CD43+ | 31 ± 6 |
| CD4+ | 20 ± 6 |
| CD8+ | 21 ± 5 |
| B220+ | 36 ± 8 |
| CD43+ | 23 ± 7 |
Mean ± SD values for three animals in each of two experiments.
DISCUSSION
We have demonstrated that day 9.0 pc yolk sac HSC contribute to definitive multilineage hematopoiesis in conditioned primary and secondary recipient animals for more than 6 months posttransplant. These results extend previous observations (1, 10, 12–15, 17, 29–31) that the day <11.0 pc yolk sac contains multipotent hematopoietic progenitors and HSC providing multilineage repopulation (in in utero or postnatal transplants) or long-term repopulation of individual hematopoietic lineages in primary adult recipients. We also report novel in vivo evidence that the pattern of yolk sac HSC differentiation is regulated in part by the hematopoietic microenvironment within which the cells reside.
Evidence to substantiate that day 9.0 yolk sac HSC solely accounted for the hematopoietic reconstitution in the conditioned newborn hosts is strengthened by the demonstration that (i) donor HSC were not contributed by maternal blood contamination since whole maternal blood did not engraft to detectable levels in the newborn hosts, (ii) the level of engraftment was donor cell dose related, and (iii) donor cell engraftment occurred in the newborn hosts whether yolk sac cells were administered intravenously or via direct liver injection. Murine hematopoietic cell transplants are generally performed by injecting donor cells into the circulation of conditioned hosts. However, it is commonly observed that only a fraction of the injected donor cells escape clearance from the circulation and seed the hematopoietic tissues (32). Because the number of donor yolk sac cells is relatively small at this stage of development (day 9.0 pc), we performed some of the transplants by directly injecting donor yolk sac cells into the newborn host liver (an easily accessible organ known to be actively producing blood cells postnatally). The higher level of donor yolk sac cell engraftment observed after the direct liver injections suggests that some yolk sac HSC are indeed lost when donor yolk sac cells are injected intravenously into the newborn hosts. These results also suggest that the newborn liver functions as a highly supportive microenvironment for engraftment of the donor yolk sac HSC.
Our observation that HSC are present in the murine yolk sac (day 9.0 pc) prior to initiation of definitive hematopoiesis in the liver (day 10.0 pc) contrasts with recent reports that HSC contributing to hepatic hematopoiesis are present at day 10.0 pc in the AGM region and at day 11.0 pc in the yolk sac (18, 21). Medvinsky and Dzierzak (21) recently cultured murine yolk sac, AGM, and liver tissue from day 9.0–11.0 pc embryos as explants in vitro and observed that HSC activity, assayed as reconstitution of the hematopoietic system of lethally irradiated adult recipients, autonomously arose on day 10.0 pc in the AGM but not in the yolk sac or liver explants. These results were interpreted to indicate that the AGM region is the first and possibly the only tissue initiating HSC development in the pre-liver stage of murine hematopoiesis. Support for this hypothesis was provided by Cumano et al. (20) who isolated paraaortic splanchnopleura (P-sp) and yolk sac tissues from embryos prior to the onset of systemic circulation of blood cells and after a 2-day organotypic culture in vitro, detected lymphoid and multipotent myeloid precursors in the P-sp explants but no lymphoid and reduced myeloid progenitor cells in the yolk sac explants. Cumano et al. (20) proposed that hematopoietic progenitor cells emerge first in the yolk sac and later in the P-sp but only hematopoietic precursors from the P-sp are endowed with the capacity to give rise to definitive hematopoietic precursors. An obvious explanation for the apparently discrepant results of the present report with those of Medvinsky and Dzierzak (21) and Cumano et al. (20) is that we transplanted yolk sac cells into conditioned newborn recipients rather than conditioned adult recipients. As a control in this study, we have also injected day 9.0 pc yolk sac cells into conditioned adult mice. We failed to detect any evidence of yolk sac cell engraftment in the adult recipients, a finding consistent with the studies performed by Medvinsky and Dzierzak (21) and Cumano et al. (20). Failure to demonstrate yolk sac cell engraftment in the adult recipients in the present studies was not due to a lack of expertise in performing bone marrow transplantation assays with small numbers of donor cells, as we have previously demonstrated the ability to engraft lethally conditioned adult recipients with limited numbers of highly enriched adult marrow HSC populations (33, 34). Rather, this observation suggests that there may be unique developmental stage specific interactions that permit embryonic HSC to interact with hematopoietic microenvironments in the newborn, but not adult host, leading to yolk sac HSC engraftment. While there may be differential expression of homing molecules and their cognate ligands in the splenic and bone marrow capillaries and/or sinusoids during murine ontogeny that could account for preferential engraftment of embryonic cells in newborn versus adult animals, we speculate that the primary difference in the level of donor cell engraftment is that the liver functions as an active hematopoietic organ, and therefore a potential homing site, in the newborn but not adult animal. At present it is unproven whether donor yolk sac HSC home first to the liver and later to the bone marrow, but it is clear that by 6 months posttransplant, donor yolk sac HSC were present in the bone marrow of the primary recipients. Further studies to determine the exact site, molecular determinants, and kinetics of yolk sac HSC homing in conditioned newborn mice should be instructive in this regard. In addition, comparative analysis of the reconstituting ability of HSC isolated from the yolk sac and P-sp at stages before onset of the systemic circulation will be required to determine if the HSC present in the day 9.0 yolk sac autonomously emerge from the yolk sac or migrate there from the P-sp.
Another remarkable feature of the donor yolk sac HSC reconstitution of the newborn recipients was that the engrafted yolk sac HSC differentiated into multiple blood cell lineages in a pattern indistinguishable from that observed in normal animals. Yolk sac cells do not normally differentiate into mature B or T lymphocytes or granulocytes in vivo in the yolk sac environment (24, 25). Nevertheless, transplantation of the yolk sac cells into a nonyolk sac hematopoietic environment in the conditioned newborn mouse resulted in appropriate homeostatic regulation of HSC differentiation into lymphocytes and granulocytes so that the number of circulating cells was not different from the distribution of cells in normal age-matched mice. These results suggest that the highly restricted differentiation and robust proliferation of the yolk sac hematopoietic cells at day 9.0 pc in situ must be regulated by the cooperative action of extrinsic and intrinsic factors (i.e., tissue morphogens, transcription factors, growth factors and/or their receptors) within the yolk sac microenvironment. Characterization of such stage- and site-specific regulatory molecules may be of interest to many investigators, as the most optimal methods for the ex vivo manipulation of murine and human HSC for gene therapy and bone marrow transplantation protocols continue to be refined to optimize engraftment and long-term expression in transduced cells.
Acknowledgments
We thank Drs. Han van der Loo, Andrew Roberts, and Rueben Kapur for critical review of the manuscript and Pat Fox for manuscript preparation. This work was supported in part by Grant 96-1057 from the March of Dimes Birth Defects Foundation.
ABBREVIATIONS
- HSC
hematopoietic stem cells
- pc
postcoitus
- AGM
aorta-gonad-mesonephros
- HBSS
Hanks’ balanced salt solution
- Gpi
glucose phosphate isomerase
References
- 1.Moore M, Metcalf D. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 2.Rugh R. The Mouse. New York: Oxford Univ. Press; 1993. pp. 116–153. [Google Scholar]
- 3.Johnson G, Jones R. J Embryol Exp Morphol. 1973;30:83–96. [PubMed] [Google Scholar]
- 4.Moore M, Owen J. Lancet. 1967;ii:658–659. [Google Scholar]
- 5.Dieterlen-Lievre F. J Embryol Exp Morphol. 1975;33:607–619. [PubMed] [Google Scholar]
- 6.Dieterlen-Lievre F, Le Douarin N. Semin Dev Biol. 1993;4:325–332. [Google Scholar]
- 7.Beaupain D, Martin C, Dieterlen-Lievre F. Blood. 1979;53:212–217. [PubMed] [Google Scholar]
- 8.Dzierzak E, Medvinsky A. Trends Genet. 1995;11:359–366. doi: 10.1016/s0168-9525(00)89107-6. [DOI] [PubMed] [Google Scholar]
- 9.Peault B. J Hematother. 1996;5:369–378. doi: 10.1089/scd.1.1996.5.369. [DOI] [PubMed] [Google Scholar]
- 10.Weissman I, Papaioannou V, Gardner R. In: Fetal Hematopoietic Origins of the Adult Hematolymphoid System. Clarkson B, Marks P A, Till J E, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1978. pp. 33–47. [Google Scholar]
- 11.Weissman I, Baird S, Gardner R, Papaioannou V, Raschke W. Cold Spring Harbor Symp Quant Biol. 1977;41:9–21. doi: 10.1101/sqb.1977.041.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Palacios R, Imhof B. Proc Natl Acad Sci USA. 1993;90:6581–6585. doi: 10.1073/pnas.90.14.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paige C, Kincade P, Moore M, Lee G. J Exp Med. 1979;150:548–563. doi: 10.1084/jem.150.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toles J F, Chui D H K, Belbeck L W, Starr E, Barker J E. Proc Natl Acad Sci USA. 1989;86:7456–7459. doi: 10.1073/pnas.86.19.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godin I, Dieterlen-Lievre F, Cumano A. Proc Natl Acad Sci USA. 1995;92:773–777. doi: 10.1073/pnas.92.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cudennec C, Thiery J, Le Douarin N. Proc Natl Acad Sci USA. 1981;78:2412–2416. doi: 10.1073/pnas.78.4.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu L, Wang S, Auerbach R. Proc Natl Acad Sci USA. 1996;93:14782–14787. doi: 10.1073/pnas.93.25.14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller A, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 19.Medvinsky A, Gan O, Semenova M, Samoylina N. Blood. 1996;87:557–566. [PubMed] [Google Scholar]
- 20.Cumano A, Dieterlen-Lievre F, Godin I. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 21.Medvinsky A, Dzierzak E. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 22.Delassus S, Cumano A. Immunity. 1996;4:97–106. doi: 10.1016/s1074-7613(00)80302-7. [DOI] [PubMed] [Google Scholar]
- 23.Wolf N, Bertoncello I, Jiang D, Priestley G. Exp Hematol. 1995;23:142–146. [PubMed] [Google Scholar]
- 24.Tavassoli M. Blood Cells. 1991;1:269–281. [PubMed] [Google Scholar]
- 25.Tavassoli M. Exp Hematol. 1994;22:7. [PubMed] [Google Scholar]
- 26.Yoder M C, Cumming J, Hiatt K, Mukherjee P, Williams D. Biol Blood Marrow Transplant. 1996;2:59–67. [PubMed] [Google Scholar]
- 27.Yoder M C, Papaioannou V, Breitfeld P, Williams D. Blood. 1994;83:2436–2443. [PubMed] [Google Scholar]
- 28.Yoder M C, King B, Hiatt K, Williams D. Blood. 1995;86:1322–1330. [PubMed] [Google Scholar]
- 29.Tyan M, Herzenberg L A. J Immunol. 1968;101:446–450. [PubMed] [Google Scholar]
- 30.Eren R, Auerbach R, Globerson A. Immunol Res. 1987;6:279–283. doi: 10.1007/BF02935522. [DOI] [PubMed] [Google Scholar]
- 31.Yoder M C, Hiatt K. Blood. 1997;86:2176–2183. [PubMed] [Google Scholar]
- 32.van der Loo J, Walentina A, Kieboom D, Ploemacher R. Blood. 1995;85:952–962. [PubMed] [Google Scholar]
- 33.Traycoff C, Cornetta K, Yoder M, Davidson A, Srour E. Exp Hematol. 1996;24:299–306. [PubMed] [Google Scholar]
- 34.Leemhuis T, Yoder M C, Grigsby S, Aguero B, Eder P, Srour E. Exp Hematol. 1996;24:1215–1224. [PubMed] [Google Scholar]