Abstract
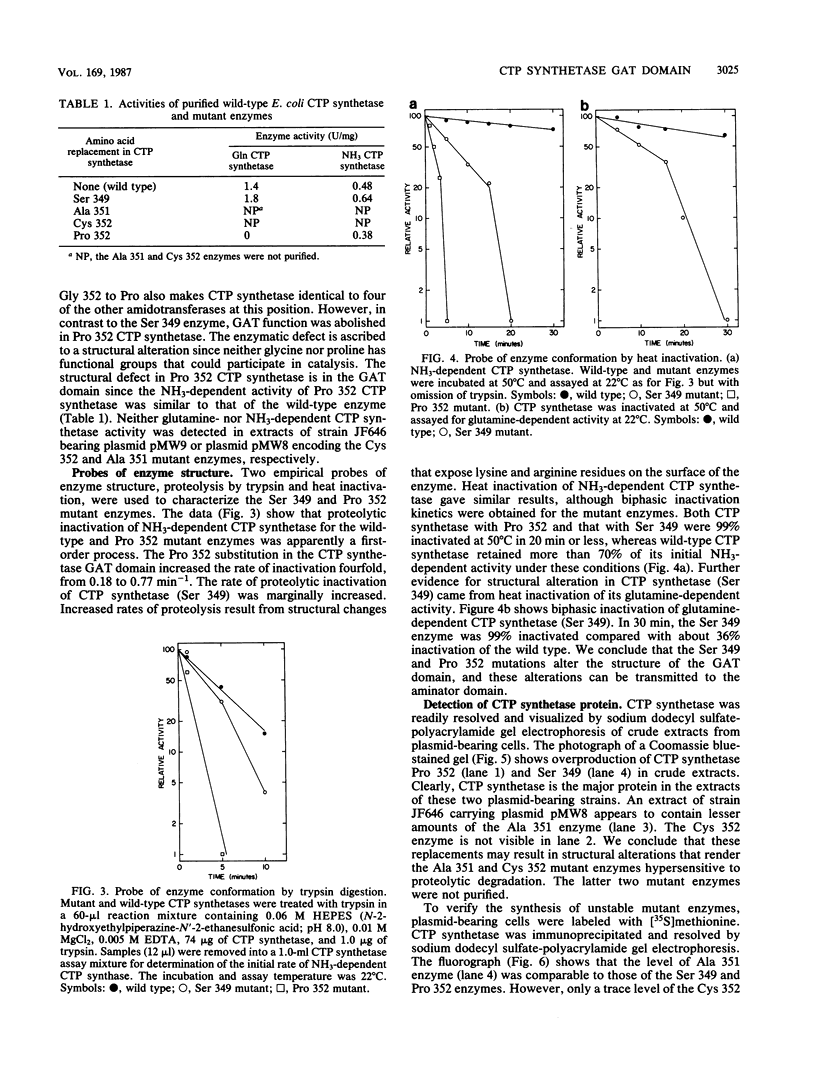
Site-directed mutations were introduced into a conserved region of the Escherichia coli CTP synthetase glutamine amide transfer domain. The amino acid replacements, valine 349 to serine, glycine 351 to alanine, glycine 352 to proline, and glycine 352 to cysteine, all increased the lability of CTP synthetase. The proline 352 replacement abolished the capacity to form the covalent glutaminyl-cysteine 379 catalytic intermediate, thus preventing glutamine amide transfer function; NH3-dependent CTP synthetase activity was retained. In CTP synthetase (serine 349), both glutamine and NH3-dependent activities were increased approximately 30% relative to that of the wild type. CTP synthetase mutants alanine 351 and cysteine 352 were not overproduced because of apparent instability and proteolytic degradation. We conclude that the conserved region between residues 346 and 355 in the CTP synthetase glutamine amide transfer domain has an important structural role.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amuro N., Paluh J. L., Zalkin H. Replacement by site-directed mutagenesis indicates a role for histidine 170 in the glutamine amide transfer function of anthranilate synthase. J Biol Chem. 1985 Nov 25;260(27):14844–14849. [PubMed] [Google Scholar]
- Anderson P. M. CTP synthetase from Escherichia coli: an improved purification procedure and characterization of hysteretic and enzyme concentration effects on kinetic properties. Biochemistry. 1983 Jun 21;22(13):3285–3292. doi: 10.1021/bi00282a038. [DOI] [PubMed] [Google Scholar]
- Bauer C. E., Hesse S. D., Waechter-Brulla D. A., Lynn S. P., Gumport R. I., Gardner J. F. A genetic enrichment for mutations constructed by oligodeoxynucleotide-directed mutagenesis. Gene. 1985;37(1-3):73–81. doi: 10.1016/0378-1119(85)90259-8. [DOI] [PubMed] [Google Scholar]
- Buchanan J. M. The amidotransferases. Adv Enzymol Relat Areas Mol Biol. 1973;39:91–183. doi: 10.1002/9780470122846.ch2. [DOI] [PubMed] [Google Scholar]
- Friesen J. D., An G., Fiil N. P. Nonsense and insertion mutants in the relA gene of E. coli: cloning relA. Cell. 1978 Dec;15(4):1187–1197. doi: 10.1016/0092-8674(78)90045-4. [DOI] [PubMed] [Google Scholar]
- Kaplan J. B., Merkel W. K., Nichols B. P. Evolution of glutamine amidotransferase genes. Nucleotide sequences of the pabA genes from Salmonella typhimurium, Klebsiella aerogenes and Serratia marcescens. J Mol Biol. 1985 Jun 5;183(3):327–340. doi: 10.1016/0022-2836(85)90004-x. [DOI] [PubMed] [Google Scholar]
- Kaplan J. B., Nichols B. P. Nucleotide sequence of Escherichia coli pabA and its evolutionary relationship to trp(G)D. J Mol Biol. 1983 Aug 15;168(3):451–468. doi: 10.1016/s0022-2836(83)80295-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Cytidine triphosphate synthetase. Covalent intermediates and mechanisms of action. Biochemistry. 1971 Aug 31;10(18):3365–3371. doi: 10.1021/bi00794a008. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Stallcup W. B., Koshland D. E., Jr Half-of-the-sites reactivity and the conformational states of cytidine triphosphate synthetase. Biochemistry. 1971 Aug 31;10(18):3371–3378. doi: 10.1021/bi00794a009. [DOI] [PubMed] [Google Scholar]
- Long C. W., Pardee A. B. Cytidine triphosphate synthetase of Escherichia coli B. I. Purification and kinetics. J Biol Chem. 1967 Oct 25;242(20):4715–4721. [PubMed] [Google Scholar]
- Makaroff C. A., Paluh J. L., Zalkin H. Mutagenesis of ligands to the [4 Fe-4S] center of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase. J Biol Chem. 1986 Aug 25;261(24):11416–11423. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nagano H., Zalkin H., Henderson E. J. The anthranilate synthetase-anthranilate-5-phosphorribosylpyrophosphate phosphoribosyltransferase aggregate. On the reaction mechanism of anthranilate synthetase from Salmonella typhimurium. J Biol Chem. 1970 Aug 10;245(15):3810–3820. [PubMed] [Google Scholar]
- Nichols B. P., Miozzari G. F., van Cleemput M., Bennett G. N., Yanofsky C. Nucleotide sequences of the trpG regions of Escherichia coli, Shigella dysenteriae, Salmonella typhimurium and Serratia marcescens. J Mol Biol. 1980 Oct 5;142(4):503–517. doi: 10.1016/0022-2836(80)90260-0. [DOI] [PubMed] [Google Scholar]
- Paluh J. L., Zalkin H., Betsch D., Weith H. L. Study of anthranilate synthase function by replacement of cysteine 84 using site-directed mutagenesis. J Biol Chem. 1985 Feb 10;260(3):1889–1894. [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Piette J., Nyunoya H., Lusty C. J., Cunin R., Weyens G., Crabeel M., Charlier D., Glansdorff N., Piérard A. DNA sequence of the carA gene and the control region of carAB: tandem promoters, respectively controlled by arginine and the pyrimidines, regulate the synthesis of carbamoyl-phosphate synthetase in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4134–4138. doi: 10.1073/pnas.81.13.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiners J. J., Jr, Zalkin H. Immunological study of anthranilate synthetase. J Bacteriol. 1975 Aug;123(2):620–630. doi: 10.1128/jb.123.2.620-630.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedeman A. A., Smith J. M., Zalkin H. Nucleotide sequence of the guaA gene encoding GMP synthetase of Escherichia coli K12. J Biol Chem. 1985 Jul 25;260(15):8676–8679. [PubMed] [Google Scholar]
- Tso J. Y., Hermodson M. A., Zalkin H. Primary structure of Serratia marcescens anthranilate synthase component II. J Biol Chem. 1980 Feb 25;255(4):1451–1457. [PubMed] [Google Scholar]
- Tso J. Y., Zalkin H., van Cleemput M., Yanofsky C., Smith J. M. Nucleotide sequence of Escherichia coli purF and deduced amino acid sequence of glutamine phosphoribosylpyrophosphate amidotransferase. J Biol Chem. 1982 Apr 10;257(7):3525–3531. [PubMed] [Google Scholar]
- Walker J. E., Gay N. J., Saraste M., Eberle A. N. DNA sequence around the Escherichia coli unc operon. Completion of the sequence of a 17 kilobase segment containing asnA, oriC, unc, glmS and phoS. Biochem J. 1984 Dec 15;224(3):799–815. doi: 10.1042/bj2240799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M., Makaroff C. A., Zalkin H. Nucleotide sequence of Escherichia coli pyrG encoding CTP synthetase. J Biol Chem. 1986 Apr 25;261(12):5568–5574. [PubMed] [Google Scholar]
- Werner M., Feller A., Piérard A. Nucleotide sequence of yeast gene CP A1 encoding the small subunit of arginine-pathway carbamoyl-phosphate synthetase. Homology of the deduced amino acid sequence to other glutamine amidotransferases. Eur J Biochem. 1985 Jan 15;146(2):371–381. doi: 10.1111/j.1432-1033.1985.tb08663.x. [DOI] [PubMed] [Google Scholar]
- Zalkin H. Anthranilate synthetase. Adv Enzymol Relat Areas Mol Biol. 1973;38:1–39. doi: 10.1002/9780470122839.ch1. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Argos P., Narayana S. V., Tiedeman A. A., Smith J. M. Identification of a trpG-related glutamine amide transfer domain in Escherichia coli GMP synthetase. J Biol Chem. 1985 Mar 25;260(6):3350–3354. [PubMed] [Google Scholar]




