Abstract
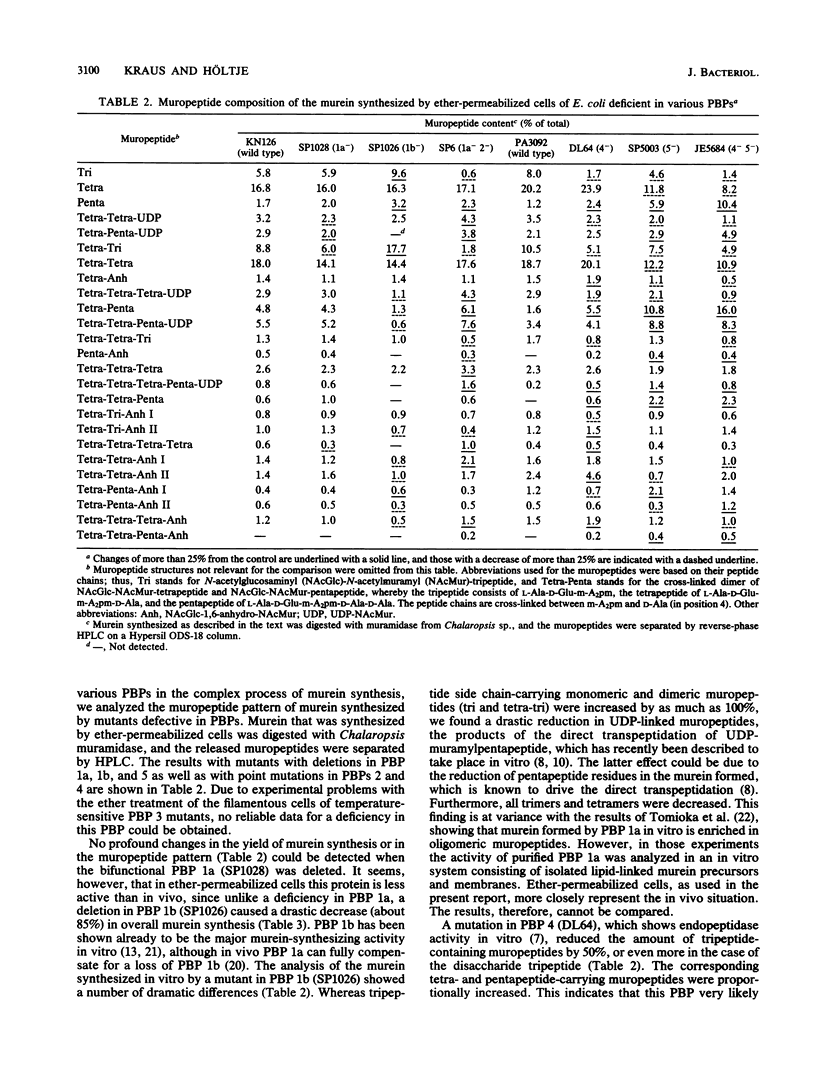
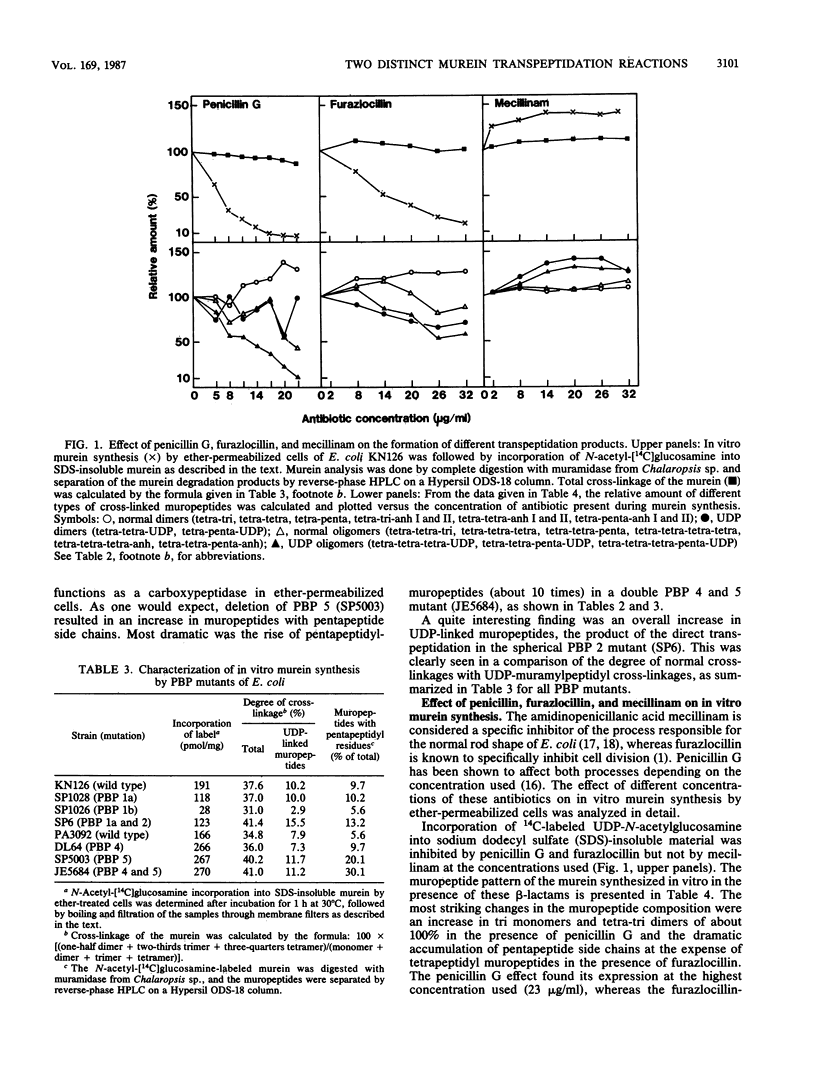
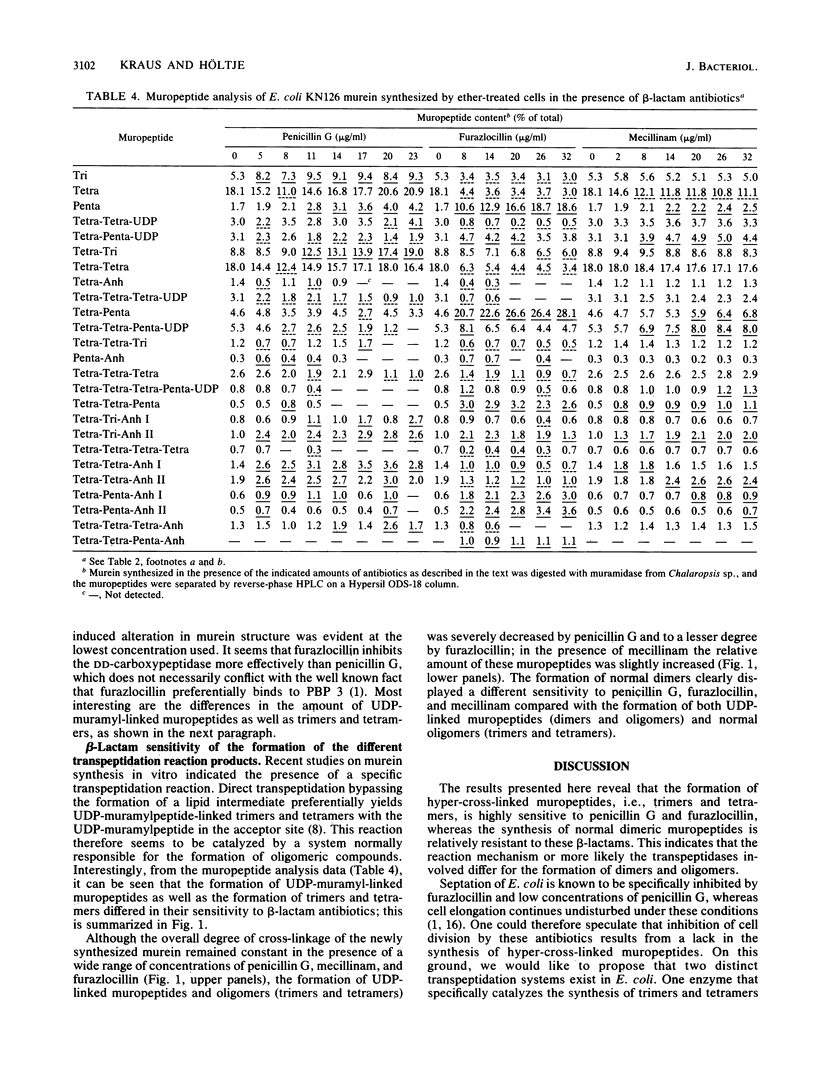
Murein synthesized in ether-permeabilized cells of Escherichia coli deficient in individual penicillin-binding proteins (PBPs) and in the presence of certain beta-lactam antibiotics was analyzed by high-pressure liquid chromatography separation of the muramidase split products. PBP 1b was found to to be the major murein synthesizing activity that was poorly compensated for by PBP 1a. A PBP 2 mutant as well as mecillinam-inhibited cells showed increased activity in the formation of oligomeric muropeptides as well as UDP-muramylpeptidyl-linked muropeptides, the reaction products of transpeptidation, bypassing the lipid intermediate. In contrast, penicillin G and furazlocillin severely inhibited these reactions but stimulated normal dimer production. It is concluded that two distinct transpeptidases exist in E. coli: one, highly sensitive to penicillin G and furazlocillin, catalyzes the formation of hyper-cross-linked muropeptides, and a second one, quite resistant to these antibiotics, synthesizes muropeptide dimers.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botta G. A., Park J. T. Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation but not during cell elongation. J Bacteriol. 1981 Jan;145(1):333–340. doi: 10.1128/jb.145.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome-Smith J. K., Edelman A., Yousif S., Spratt B. G. The nucleotide sequences of the ponA and ponB genes encoding penicillin-binding protein 1A and 1B of Escherichia coli K12. Eur J Biochem. 1985 Mar 1;147(2):437–446. doi: 10.1111/j.1432-1033.1985.tb08768.x. [DOI] [PubMed] [Google Scholar]
- Ishino F., Matsuhashi M. Peptidoglycan synthetic enzyme activities of highly purified penicillin-binding protein 3 in Escherichia coli: a septum-forming reaction sequence. Biochem Biophys Res Commun. 1981 Aug 14;101(3):905–911. doi: 10.1016/0006-291x(81)91835-0. [DOI] [PubMed] [Google Scholar]
- Ishino F., Tamaki S., Spratt B. G., Matsuhashi M. A mecillinam-sensitive peptidoglycan crosslinking reaction in Escherichia coli. Biochem Biophys Res Commun. 1982 Dec 15;109(3):689–696. doi: 10.1016/0006-291x(82)91995-7. [DOI] [PubMed] [Google Scholar]
- Keck W., Schwarz U. Escherichia coli murein-DD-endopeptidase insensitive to beta-lactam antibiotics. J Bacteriol. 1979 Sep;139(3):770–774. doi: 10.1128/jb.139.3.770-774.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus W., Glauner B., Höltje J. V. UDP-N-acetylmuramylpentapeptide as acceptor in murein biosynthesis in Escherichia coli membranes and ether-permeabilized cells. J Bacteriol. 1985 Jun;162(3):1000–1004. doi: 10.1128/jb.162.3.1000-1004.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass D., Pelzer H. Murein biosynthesis in ether permeabilized Escherichia coli starting from early peptidoglycan precursors. Arch Microbiol. 1981 Dec;130(4):301–306. doi: 10.1007/BF00425944. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Takagaki Y., Maruyama I. N., Tamaki S., Nishimura Y., Suzuki H., Ogino U., Hirota Y. Mutants of Escherichia coli lacking in highly penicillin-sensitive D-alanine carboxypeptidase activity. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2976–2979. doi: 10.1073/pnas.74.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Horiuchi T. Isolation and characterization of a temperature-sensitive amber suppressor mutant of Escherichia coli K12. Mol Gen Genet. 1973;123(1):77–88. doi: 10.1007/BF00282991. [DOI] [PubMed] [Google Scholar]
- Nakagawa J., Matsuhashi M. Molecular divergence of a major peptidoglycan synthetase with transglycosylase-transpeptidase activities in Escherichia coli --- penicillin-binding protein 1Bs. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1546–1553. doi: 10.1016/0006-291x(82)90964-0. [DOI] [PubMed] [Google Scholar]
- Park J. T., Burman L. FL-1060: a new penicillin with a unique mode of action. Biochem Biophys Res Commun. 1973 Apr 16;51(4):863–868. doi: 10.1016/0006-291x(73)90006-5. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Deletion of the penicillin-binding protein 5 gene of Escherichia coli. J Bacteriol. 1980 Dec;144(3):1190–1192. doi: 10.1128/jb.144.3.1190-1192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., van Heijenoort Y., Tamura T., Mizoguchi J., Hirota Y., van Heijenoort J. In vitro peptidoglycan polymerization catalysed by penicillin binding protein 1b of Escherichia coli K-12. FEBS Lett. 1980 Feb 11;110(2):245–249. doi: 10.1016/0014-5793(80)80083-4. [DOI] [PubMed] [Google Scholar]
- Tomioka S., Ishino F., Tamaki S., Matsuhashi M. Formation of hyper-crosslinked peptidoglycan with multiple crosslinkages by a penicillin-binding protein, 1A, of Escherichia coli. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1175–1182. doi: 10.1016/0006-291x(82)91236-0. [DOI] [PubMed] [Google Scholar]
- Yousif S. Y., Broome-Smith J. K., Spratt B. G. Lysis of Escherichia coli by beta-lactam antibiotics: deletion analysis of the role of penicillin-binding proteins 1A and 1B. J Gen Microbiol. 1985 Oct;131(10):2839–2845. doi: 10.1099/00221287-131-10-2839. [DOI] [PubMed] [Google Scholar]


