Abstract
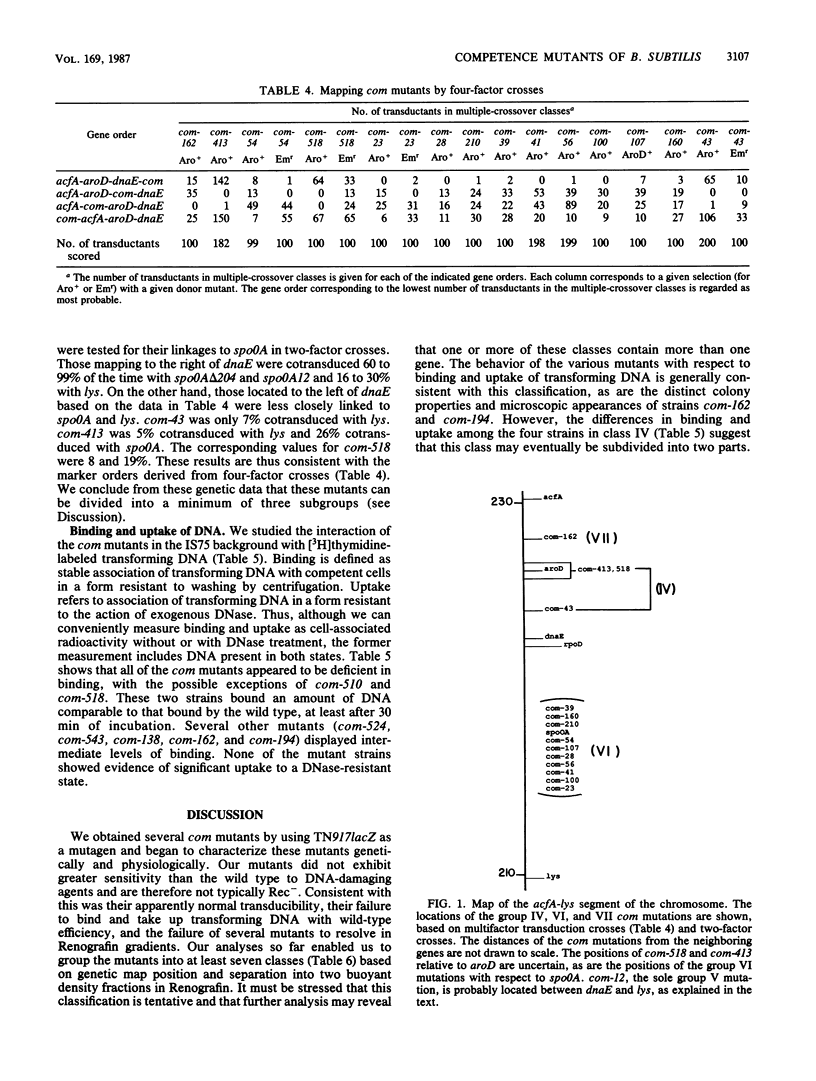
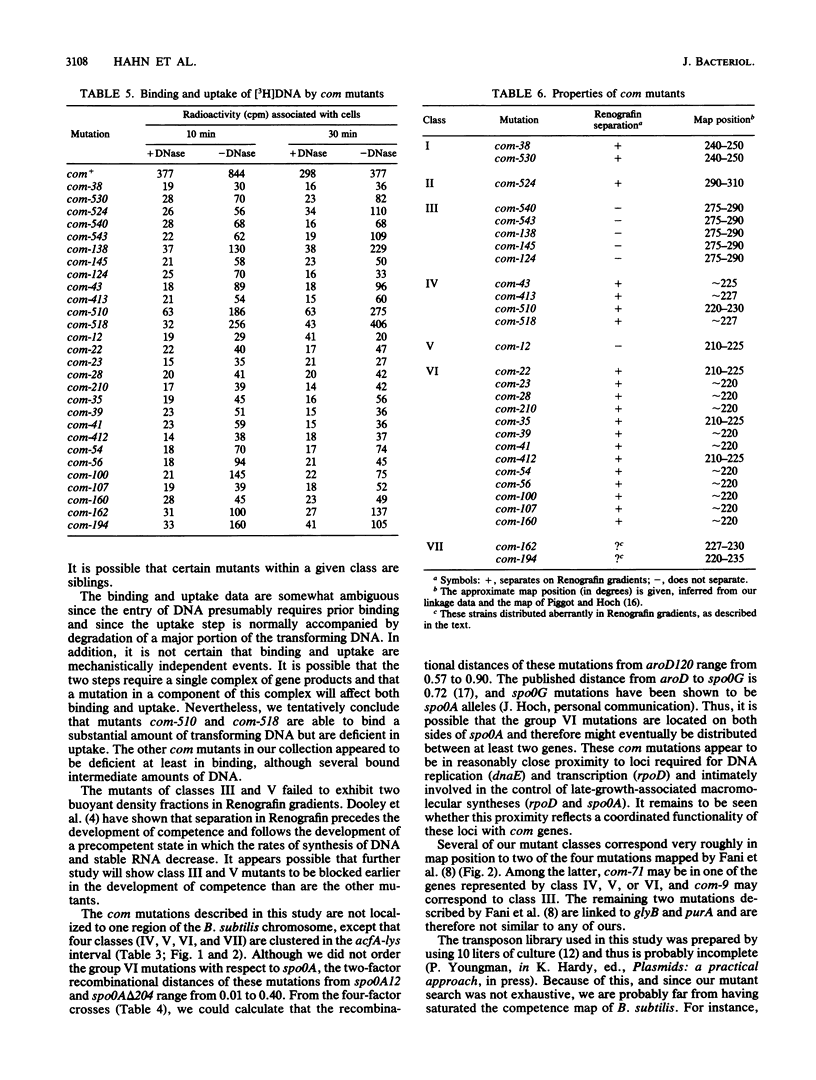
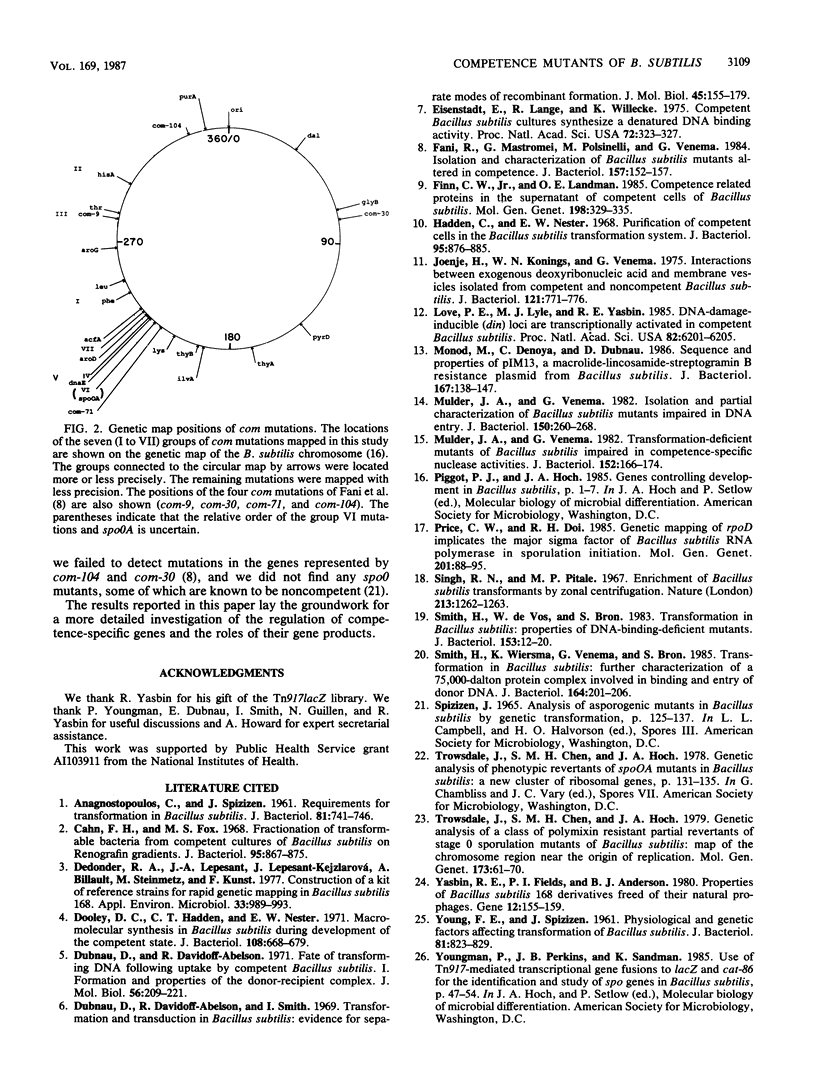
We isolated 28 mutants of Bacillus subtilis deficient in the development of competence by using the transposon Tn917lacZ as a mutagen. The mutant strains were poorly transformable with plasmid and chromosomal DNAs but were normally transducible and exhibited wild-type resistance to DNA-damaging agents. The mutations were genetically mapped, and the mutants were characterized with respect to their abilities to bind and take up radiolabeled DNA. All were defective in uptake, and some failed to bind significant amounts of DNA. The abilities of the mutant strains to resolve into two buoyant density classes on Renografin gradients were studied. Most resolved normally, but several banded in Renografin only at the buoyant density of noncompetent cells. The genetic mapping studies and the other analyses suggested that the mutations define a minimum of seven distinct com genes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn F. H., Fox M. S. Fractionation of transformable bacteria from ocompetent cultures of Bacillus subtilis on renografin gradients. J Bacteriol. 1968 Mar;95(3):867–875. doi: 10.1128/jb.95.3.867-875.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedonder R. A., Lepesant J. A., Lepesant-Kejzlarová J., Billault A., Steinmetz M., Kunst F. Construction of a kit of reference strains for rapid genetic mapping in Bacillus subtilis 168. Appl Environ Microbiol. 1977 Apr;33(4):989–993. doi: 10.1128/aem.33.4.989-993.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley D. C., Hadden C. T., Nester E. W. Macromolecular synthesis in Bacillus subtilis during development of the competent state. J Bacteriol. 1971 Nov;108(2):668–679. doi: 10.1128/jb.108.2.668-679.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Smith I. Transformation and transduction in Bacillus subtilis: evidence for separate modes of recombinant formation. J Mol Biol. 1969 Oct 28;45(2):155–179. doi: 10.1016/0022-2836(69)90097-7. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Lange R., Willecke K. Competent Bacillus subtilis cultures synthesize a denatured DNA binding activity. Proc Natl Acad Sci U S A. 1975 Jan;72(1):323–327. doi: 10.1073/pnas.72.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani R., Mastromei G., Polsinelli M., Venema G. Isolation and characterization of Bacillus subtilis mutants altered in competence. J Bacteriol. 1984 Jan;157(1):152–157. doi: 10.1128/jb.157.1.152-157.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn C. W., Jr, Landman O. E. Competence related proteins in the supernatant of competent cells of Bacillus subtilis. Mol Gen Genet. 1985;198(2):329–335. doi: 10.1007/BF00383015. [DOI] [PubMed] [Google Scholar]
- Hadden C., Nester E. W. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968 Mar;95(3):876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joenje H., Konings W. N., Venema G. Interactions between exogenous deoxyribonucleic acid and membrane vesicles isolated from competent and noncompetent Bacillus subtilis. J Bacteriol. 1975 Mar;121(3):771–776. doi: 10.1128/jb.121.3.771-776.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love P. E., Lyle M. J., Yasbin R. E. DNA-damage-inducible (din) loci are transcriptionally activated in competent Bacillus subtilis. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6201–6205. doi: 10.1073/pnas.82.18.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod M., Denoya C., Dubnau D. Sequence and properties of pIM13, a macrolide-lincosamide-streptogramin B resistance plasmid from Bacillus subtilis. J Bacteriol. 1986 Jul;167(1):138–147. doi: 10.1128/jb.167.1.138-147.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J. A., Venema G. Isolation and partial characterization of Bacillus subtilis mutants impaired in DNA entry. J Bacteriol. 1982 Apr;150(1):260–268. doi: 10.1128/jb.150.1.260-268.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J. A., Venema G. Transformation-deficient mutants of Bacillus subtilis impaired in competence-specific nuclease activities. J Bacteriol. 1982 Oct;152(1):166–174. doi: 10.1128/jb.152.1.166-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. W., Doi R. H. Genetic mapping of rpoD implicates the major sigma factor of Bacillus subtilis RNA polymerase in sporulation initiation. Mol Gen Genet. 1985;201(1):88–95. doi: 10.1007/BF00397991. [DOI] [PubMed] [Google Scholar]
- Smith H., Wiersma K., Venema G., Bron S. Transformation in Bacillus subtilis: further characterization of a 75,000-dalton protein complex involved in binding and entry of donor DNA. J Bacteriol. 1985 Oct;164(1):201–206. doi: 10.1128/jb.164.1.201-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., de Vos W., Bron S. Transformation in Bacillus subtilis: properties of DNA-binding-deficient mutants. J Bacteriol. 1983 Jan;153(1):12–20. doi: 10.1128/jb.153.1.12-20.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J., Chen S. M., Hoch J. A. Genetic analysis of a class of polymyxin resistant partial revertants of stage O sporulation mutants of Bacillus subtilis: map of the chromosome region near the origin of replication. Mol Gen Genet. 1979 May 23;173(1):61–70. doi: 10.1007/BF00267691. [DOI] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. Physiological and genetic factors affecting transformation of Bacillus subtilis. J Bacteriol. 1961 May;81:823–829. doi: 10.1128/jb.81.5.823-829.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Fields P. I., Andersen B. J. Properties of Bacillus subtilis 168 derivatives freed of their natural prophages. Gene. 1980 Dec;12(1-2):155–159. doi: 10.1016/0378-1119(80)90026-8. [DOI] [PubMed] [Google Scholar]


