Abstract
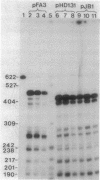
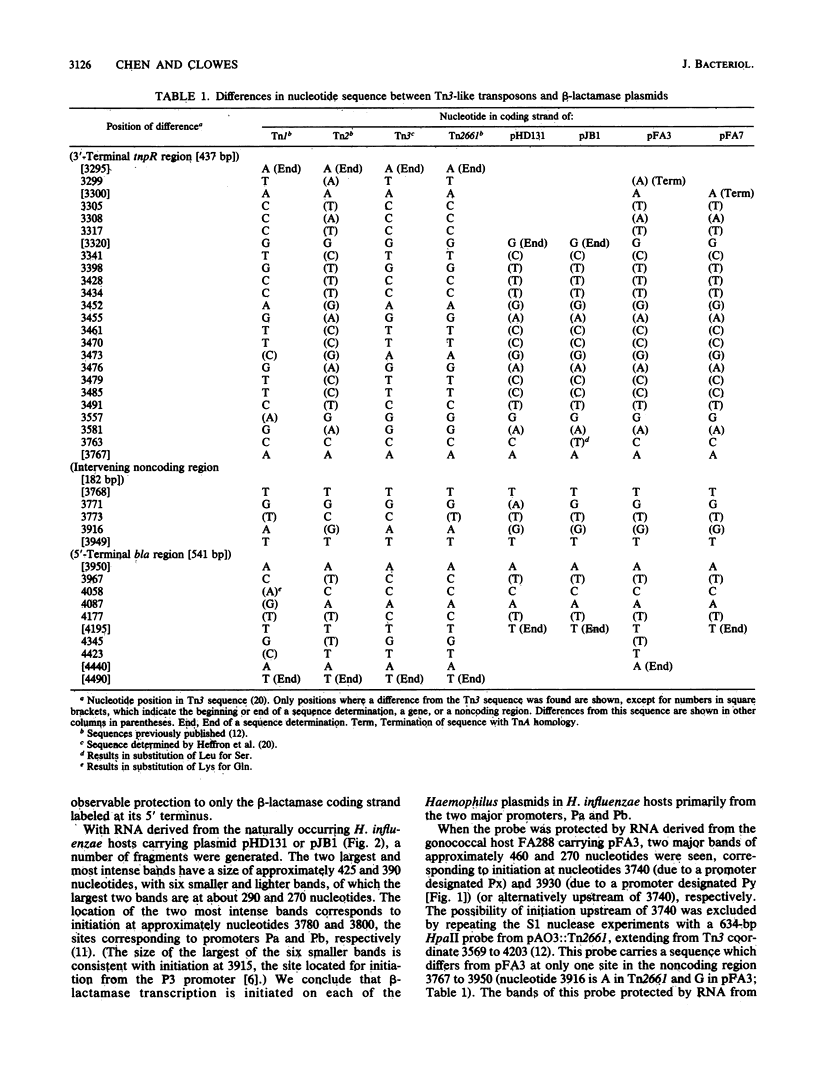
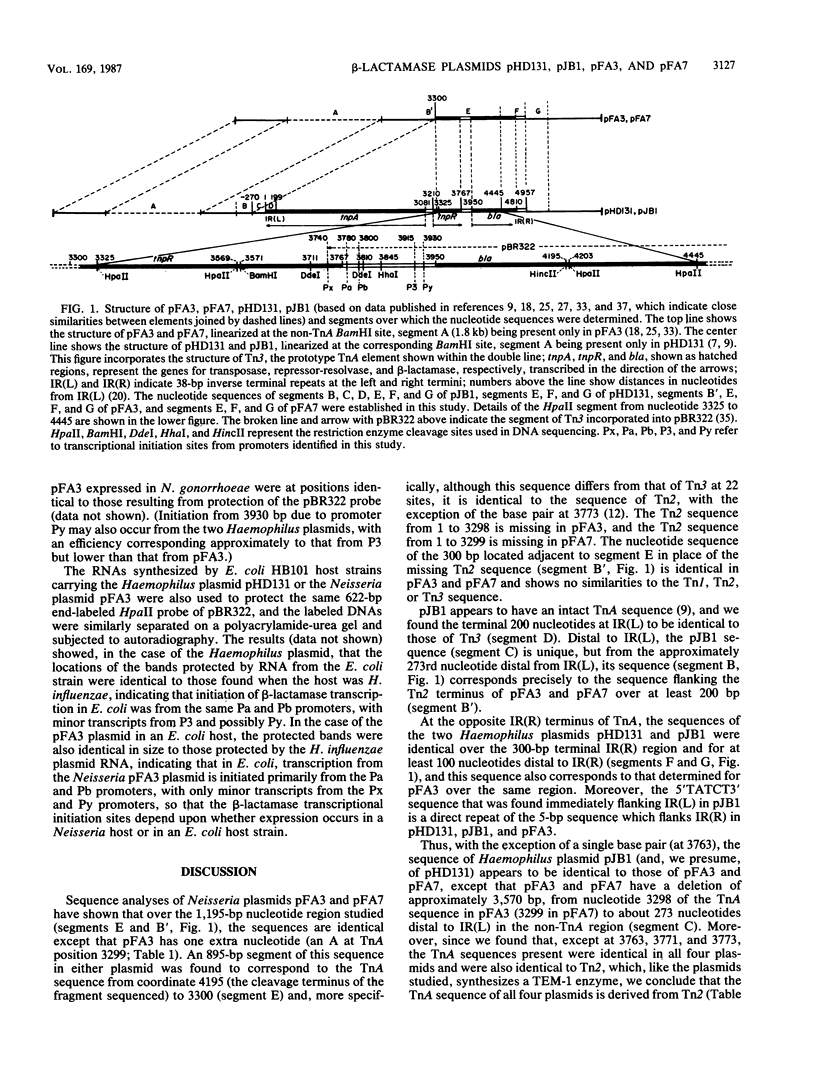
The sites of initiation for beta-lactamase mRNA transcription and the nucleotide sequences of beta-lactamase plasmids derived from Haemophilus and Neisseria species were determined. In N. gonorrhoeae, transcription from plasmid pFA3 was initiated from two sites, one located about 20 base pairs (bp) and the other 210 bp upstream of the beta-lactamase initiating codon, whereas in H. influenzae, transcriptional initiation from plasmid pHD131 occurred at two different sites, approximately 150 and 170 bp upstream of the initiating codon. When these plasmids were transformed into Escherichia coli, transcription was initiated at the 150- and 170-bp upstream sites in both plasmids. The nucleotide sequences of both plasmids within the noncoding region upstream of the transcriptional initiation site were identical and, except at two or three nucleotide positions, the sequences were also identical to the corresponding region of Tn3. At one of these positions there is a TA for CG substitution, which correlates in E. coli and Haemophilus sp. with the presence of two strong, overlapping beta-lactamase promoters, initiating transcription at the 150- and 170-bp upstream sites. Over a larger (875-bp) segment comprising most of the sequences of the tnpR and bla genes, the nucleotide sequences of both plasmids were also identical, and although this sequence differed from the corresponding Tn3 sequence at 18 sites, it was identical to that of Tn2, except at one site. The sequence of a second Haemophilus plasmid, pJB1, was identical to that of pHD131 in the same region, except at two nucleotides. All three plasmids were identical in nucleotide sequence in other TnA regions, as well as in regions flanking the TnA sequence, except that the Neisseria plasmid lacked a TnA segment of 3,298 bp [comprising the IR(L) and proximal sequences] together with approximately 273 bp of the non-TnA region adjacent to IR(L). The sequence of a second N. gonorrhoeae plasmid, pFA7, was identical to pFA3, except that the terminal, 3,299 TnA nucleotides were missing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brosius J., Cate R. L., Perlmutter A. P. Precise location of two promoters for the beta-lactamase gene of pBR322. S1 mapping of ribonucleic acid isolated from Escherichia coli or synthesized in vitro. J Biol Chem. 1982 Aug 10;257(15):9205–9210. [PubMed] [Google Scholar]
- Brunton J., Bennett P., Grinsted J. Molecular nature of a plasmid specifying beta-lactamase production in Haemophilus ducreyi. J Bacteriol. 1981 Dec;148(3):788–795. doi: 10.1128/jb.148.3.788-795.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J., Meier M., Ehrman N., Maclean I., Slaney L., Albritton W. L. Molecular epidemiology of beta-lactamase-specifying plasmids of Haemophilus ducreyi. Antimicrob Agents Chemother. 1982 Jun;21(6):857–863. doi: 10.1128/aac.21.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J., Meier M., Erhman N., Clare D., Almawy R. Origin of small beta-lactamase-specifying plasmids in Haemophilus species and Neisseria gonorrhoeae. J Bacteriol. 1986 Oct;168(1):374–379. doi: 10.1128/jb.168.1.374-379.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. T., Clowes R. C. Two improved promoter sequences for the beta-lactamase expression arising from a single base-pair substitution. Nucleic Acids Res. 1984 Apr 11;12(7):3219–3234. doi: 10.1093/nar/12.7.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. T., Clowes R. C. Variations between the nucleotide sequences of Tn1, Tn2, and Tn3 and expression of beta-lactamase in Pseudomonas aeruginosa and Escherichia coli. J Bacteriol. 1987 Feb;169(2):913–916. doi: 10.1128/jb.169.2.913-916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang S. J., Clowes R. C. Intramolecular transposition and inversion in plasmid R6K. J Bacteriol. 1980 May;142(2):668–682. doi: 10.1128/jb.142.2.668-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaff J., Elwell L. P., Falkow S. Molecular nature of two beta-lactamase-specifying plasmids isolated from Haemophilus influenzae type b. J Bacteriol. 1976 Apr;126(1):439–446. doi: 10.1128/jb.126.1.439-446.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd H. M., Bennett P. M. R46 encodes a site-specific recombination system interchangeable with the resolution function of TnA. Plasmid. 1983 May;9(3):247–261. doi: 10.1016/0147-619x(83)90003-3. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Sox T., Biswas G., Blackman E., Sparling P. F. Conjugal transfer of the gonococcal penicillinase plasmid. Science. 1977 Mar 11;195(4282):998–1000. doi: 10.1126/science.402693. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayet O., Froment Y., Piffaretti J. C. Beta-lactamase-specifying plasmids isolated from Neisseria gonorrhoeae have retained an intact right part of a Tn3-like transposon. J Bacteriol. 1982 Jan;149(1):136–144. doi: 10.1128/jb.149.1.136-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Pero J. Cascades of Sigma factors. Cell. 1981 Sep;25(3):582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. W., Robbins K. E. Evolutionary analysis of the 7.1-kb beta-lactamase-specifying R-plasmid of Neisseria gonorrhoeae by restriction endonucleases. J Bacteriol. 1983 Jun;154(3):1498–1501. doi: 10.1128/jb.154.3.1498-1501.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- McNicol P. J., Albritton W. L., Ronald A. R. Characterization of ampicillin resistance plasmids of Haemophilus ducreyi and Neisseria gonorrhoeae with regard to location of origin of transfer and mobilization by a conjugative plasmid of Haemophilus ducreyi. J Bacteriol. 1983 Oct;156(1):437–440. doi: 10.1128/jb.156.1.437-440.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Roberts M., Elwell L. P., Falkow S. Molecular characterization of two beta-lactamase-specifying plasmids isolated from Neisseria gonorrhoeae. J Bacteriol. 1977 Aug;131(2):557–563. doi: 10.1128/jb.131.2.557-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Blackman E., Sparling P. F. Genetic mapping of linked antibiotic resistance loci in Neisseria gonorrhoeae. J Bacteriol. 1974 Dec;120(3):1284–1292. doi: 10.1128/jb.120.3.1284-1292.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sox T. E., Mohammed W., Sparling P. F. Transformation-derived Neisseria gonorrhoeae plasmids with altered structure and function. J Bacteriol. 1979 May;138(2):510–518. doi: 10.1128/jb.138.2.510-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Thorpe P. A., Clowes R. C. Absence of direct repeats flanking transposons resulting from intramolecular transposition. Gene. 1984 Apr;28(1):103–112. doi: 10.1016/0378-1119(84)90092-1. [DOI] [PubMed] [Google Scholar]
- Westpheling J., Ranes M., Losick R. RNA polymerase heterogeneity in Streptomyces coelicolor. Nature. 1985 Jan 3;313(5997):22–27. doi: 10.1038/313022a0. [DOI] [PubMed] [Google Scholar]
- van Embden J. D., van Klingeren B., Dessens-Kroon M., van Wijngaarden L. J. Penicillinase-producing Neisseria gonorrhoeae in the Netherlands: epidemiology and genetic and molecular characterization of their plasmids. Antimicrob Agents Chemother. 1980 Nov;18(5):789–797. doi: 10.1128/aac.18.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]