Abstract
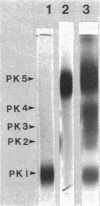
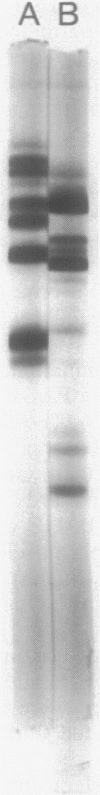
The dimorphic phycomycete Mucor racemosus was found to contain up to five electrophoretic forms of pyruvate kinase (ATP: pyruvate 2-O-phosphotransferase, EC 2.7.1.40) depending on growth conditions. M. racemosus hyphal cells grown on glutamic acid as the carbon source contained only the fastest electrophoretic form, designated PK1, while yeast cells grown on glucose contained only the slowest electrophoretic form, PK5. Intermediate electrophoretic forms PK2, PK3, and PK4 as well as PK1 and PK5 were found in hyphal cells grown on media containing fructose or cellibiose. All five electrophoretic forms had molecular weights of ca. 230,000 as determined from plots of log Rm versus acrylamide gel concentration. Both PK1 and PK5 were purified to homogeneity and determined to be homotetramers, with subunit molecular weights of 54,000 and 58,100, respectively. The amino acid content of PK1 and PK5 was determined and found to be similar but not identical. Analysis of limited tryptic digests and cyanogen bromide cleavage fragments of PK1 and PK5 indicate that the subunits of the two isozymes are significantly different.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI-GARCIA S., NICKERSON W. J. Nutrition, growth, and morphogenesis of Mucor rouxii. J Bacteriol. 1962 Oct;84:841–858. doi: 10.1128/jb.84.4.841-858.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Control of dimorphism in Mucor by hexoses: inhibition of hyphal morphogenesis. J Bacteriol. 1968 Nov;96(5):1586–1594. doi: 10.1128/jb.96.5.1586-1594.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borun T. W., Ajiro K., Zweidler A., Dolby T. W., Stephens R. E. Studies of human histone messenger RNA. II. The resolution of fractions containing individual human histone messenger RNA species. J Biol Chem. 1977 Jan 10;252(1):173–180. [PubMed] [Google Scholar]
- Cardenas J. M., Dyson R. D. Mammalian pyruvate kinase hybrid isozymes: tissue distribution and physiological significance. J Exp Zool. 1978 Jun;204(3):361–367. doi: 10.1002/jez.1402040307. [DOI] [PubMed] [Google Scholar]
- Elmer G. W., Nickerson W. J. Nutritional requirements for growth and yeastlike development of Mucor rouxii under carbon dioxide. J Bacteriol. 1970 Feb;101(2):595–602. doi: 10.1128/jb.101.2.595-602.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenthal M., Roselino E., Passeron S. Multiple molecular forms of pyruvate kinase from Mucor rouxii. Immunological relationship among the three isoenzymes and nutritional factors affecting the enzymatic pattern. Eur J Biochem. 1973 May;35(1):148–158. doi: 10.1111/j.1432-1033.1973.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Guderley H. E., Cardenas J. M. Developmental changes in the pyruvate kinase isozymes of coho salmon. J Exp Zool. 1979 Apr;208(1):1–12. doi: 10.1002/jez.1402080102. [DOI] [PubMed] [Google Scholar]
- Hall E. R., Cottam G. L. Isozymes of pyruvate kinase in vertebrates: their physical, chemical, kinetic and immunological properties. Int J Biochem. 1978;9(11):785–793. doi: 10.1016/0020-711x(78)90027-7. [DOI] [PubMed] [Google Scholar]
- Hall E. R., McCully V., Cottam G. L. Evidence for a proteolytic modification of liver pyruvate kinase in fasted rats. Arch Biochem Biophys. 1979 Jul;195(2):315–324. doi: 10.1016/0003-9861(79)90357-6. [DOI] [PubMed] [Google Scholar]
- Harada K., Saheki S., Wada K., Tanaka T. Purification of four pyruvate kinase isozymes of rats by affinity elution chromatography. Biochim Biophys Acta. 1978 Jun 9;524(2):327–339. doi: 10.1016/0005-2744(78)90169-9. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hunsley J. R., Suelter C. H. Yeast pyruvate kinase. I. Purification and some chemical properties. J Biol Chem. 1969 Sep 25;244(18):4815–4818. [PubMed] [Google Scholar]
- LELOIR L. F., GOLDEMBERG S. H. Synthesis of glycogen from uridine diphosphate glucose in liver. J Biol Chem. 1960 Apr;235:919–923. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malcovati M., Kornberg H. L. Two types of pyruvate kinase in Escherichia coli K12. Biochim Biophys Acta. 1969 Apr 22;178(2):420–423. doi: 10.1016/0005-2744(69)90417-3. [DOI] [PubMed] [Google Scholar]
- O'Brien M. D., Kapoor M. Studies of the structure-function relationships of Neurospora pyruvate kinase: denaturation by urea and the effect of ligands on the refolding and renaturation process. Int J Biochem. 1980;11(2):107–116. doi: 10.1016/0020-711x(80)90242-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Passeron Susana, Roselino Eduardo. A new form of pyruvate kinase in mycelium of Mucor rouxii. FEBS Lett. 1971 Oct 15;18(1):9–12. doi: 10.1016/0014-5793(71)80394-0. [DOI] [PubMed] [Google Scholar]
- Paznokas J. L., Sypherd P. S. Pyruvate kinase isozymes of Mucor racemosus: control of synthesis by glucose. J Bacteriol. 1977 May;130(2):661–666. doi: 10.1128/jb.130.2.661-666.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertierra A. G., Cooper R. A. Pyruvate formation during the catabolism of simple hexose sugars by Escherichia coli: studies with pyruvate kinase-negative mutants. J Bacteriol. 1977 Mar;129(3):1208–1214. doi: 10.1128/jb.129.3.1208-1214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandholm J. J., Dyson R. D., Cardenas J. M. Bovine pyruvate kinase isozymes and hybrid isozymes. Electrophoretic studies and tissue distribution. Arch Biochem Biophys. 1976 Mar;173(1):125–131. doi: 10.1016/0003-9861(76)90242-3. [DOI] [PubMed] [Google Scholar]
- Valentini G., Iadarola P., Somani B. L., Malcovati M. Two forms of pyruvate kinase in Escherichia coli. A comparison of chemical and molecular properties. Biochim Biophys Acta. 1979 Oct 11;570(2):248–258. doi: 10.1016/0005-2744(79)90145-1. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Rayman M. K., Sanwal B. D. The control of pyruvate kinases of Escherichia coli. II. Effectors and regulatory properties of the enzyme activated by ribose 5-phosphate. Can J Biochem. 1975 Apr;53(4):444–454. doi: 10.1139/o75-061. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Sanwal B. D. The control of pyruvate kinases of Escherichia coli. I. Physicochemical and regulatory properties of the enzyme activated by fructose 1,6-diphosphate. J Biol Chem. 1974 Jan 10;249(1):265–274. [PubMed] [Google Scholar]