Abstract
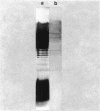
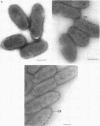
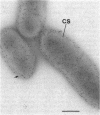
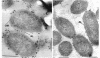
Two lipopolysaccharide O-antigen-specific monoclonal antibodies, MA1-8 (an immunoglobulin G1 [IgG1]) and MF15-4 (an IgM), were used to localize the O antigen of the lipopolysaccharide of Pseudomonas aeruginosa PAO1. A protein A-dextran-gold conjugate with an average particle diameter of 12.5 nm was used to label bacterial cells treated with MA1-8, while a second antibody (goat anti-mouse IgM) was required before the same probe could interact with cells treated with the IgM antibody MF15-4. Both antibodies resulted in exclusive labeling of the surface of P. aeruginosa PAO1 but not that of an isogenic O-antigen-lacking rough mutant. When the monoclonal antibodies became attached to the cell surface of P. aeruginosa PAO1, resulting in an even coating, the foldings and other topographic details could not be discerned by negative staining. In thin sections of monoclonal-antibody-treated bacteria, a 20- and a 30- to 40-nm thick amorphous layer was observed around the outside of the outer membrane when MA1-8 (IgG) and MF15-4 (IgM) plus goat anti-mouse IgM antibodies were used, respectively. This amorphous layer presumably resulted from the stabilization of the lipopolysaccharide structure by the monoclonal antibodies which prevented the long O-antigen chains from collapsing owing to dehydration.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendayan M. Double immunocytochemical labeling applying the protein A-gold technique. J Histochem Cytochem. 1982 Jan;30(1):81–85. doi: 10.1177/30.1.6172469. [DOI] [PubMed] [Google Scholar]
- Berry D., Kropinski A. M. Effect of lipopolysaccharide mutations and temperature on plasmid transformation efficiency in Pseudomonas aeruginosa. Can J Microbiol. 1986 May;32(5):436–438. doi: 10.1139/m86-082. [DOI] [PubMed] [Google Scholar]
- Birdsell D. C., Doyle R. J., Morgenstern M. Organization of teichoic acid in the cell wall of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):726–734. doi: 10.1128/jb.121.2.726-734.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R., Lam J. S., Lam K., Costerton J. W. Influence of culture conditions on expression of the mucoid mode of growth of Pseudomonas aeruginosa. J Clin Microbiol. 1984 Jan;19(1):8–16. doi: 10.1128/jcm.19.1.8-16.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Pitt T. L., Fürer E., Germanier R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect Immun. 1984 May;44(2):508–513. doi: 10.1128/iai.44.2.508-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett R. G., Harrison G. M., Carter R. E. Mucoid Pseudomonas aeruginosa in patients with chronic illnesses. Lancet. 1971 Jan 30;1(7692):236–237. doi: 10.1016/s0140-6736(71)90973-1. [DOI] [PubMed] [Google Scholar]
- Goodman S. L., Hodges G. M., Livingston D. C. A review of the colloidal gold marker system. Scan Electron Microsc. 1980;(Pt 2):133–146. [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Mouat E. C. Immunotherapeutic potential of monoclonal antibodies against Pseudomonas aeruginosa protein F. Eur J Clin Microbiol. 1985 Apr;4(2):224–227. doi: 10.1007/BF02013602. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Wieczorek A. A., Mutharia L. M., Poole K. Monoclonal antibodies against Pseudomonas aeruginosa outer membrane antigens: isolation and characterization. Infect Immun. 1982 Jul;37(1):166–171. doi: 10.1128/iai.37.1.166-171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks D., Molday R. S. Differential immunogold-dextran labeling of bovine and frog rod and cone cells using monoclonal antibodies against bovine rhodopsin. Exp Eye Res. 1986 Jan;42(1):55–71. doi: 10.1016/0014-4835(86)90017-5. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Beveridge T. J. Electron microscopy: its development and application to microbiology. Can J Microbiol. 1982 Jan;28(1):1–53. doi: 10.1139/m82-001. [DOI] [PubMed] [Google Scholar]
- Horisberger M. Colloidal gold : a cytochemical marker for light and fluorescent microscopy and for transmission and scanning electron microscopy. Scan Electron Microsc. 1981;(Pt 2):9–31. [PubMed] [Google Scholar]
- Knirel Y. A., Vinogradov E. V., Shashkov A. S., Dmitriev B. A., Kochetkov N. K., Stanislavsky E. S., Mashilova G. M. Somatic antigens of Pseudomonas aeruginosa. The structure of O-specific polysaccharide chains of P. aeruginosa O:3a, b and O:3a, d lipopolysaccharides. Eur J Biochem. 1982 Nov;128(1):81–90. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lam J. S., MacDonald L. A., Lam M. Y., Duchesne L. G., Southam G. G. Production and characterization of monoclonal antibodies against serotype strains of Pseudomonas aeruginosa. Infect Immun. 1987 May;55(5):1051–1057. doi: 10.1128/iai.55.5.1051-1057.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J. S., Mutharia L. M., Hancock R. E., Høiby N., Lam K., Baek L., Costerton J. W. Immunogenicity of Pseudomonas aeruginosa outer membrane antigens examined by crossed immunoelectrophoresis. Infect Immun. 1983 Oct;42(1):88–98. doi: 10.1128/iai.42.1.88-98.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmark R., Thorén-Tolling K., Sjöquist J. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J Immunol Methods. 1983 Aug 12;62(1):1–13. doi: 10.1016/0022-1759(83)90104-7. [DOI] [PubMed] [Google Scholar]
- MacaAlister T. J., Irvin R. T., Costerton J. W. Cell surface-localized alkaline phosphatase of Escherichia coli as visualized by reaction product deposition and ferritin-labeled antibodies. J Bacteriol. 1977 Apr;130(1):318–328. doi: 10.1128/jb.130.1.318-328.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie M. R., Warner N. L., Mitchell G. F. The binding of murine immunoglobulins to staphylococcal protein A. J Immunol. 1978 May;120(5):1493–1496. [PubMed] [Google Scholar]
- Mulford C. A., Osborn M. J. An intermediate step in translocation of lipopolysaccharide to the outer membrane of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1159–1163. doi: 10.1073/pnas.80.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Hancock R. E. Surface localization of Pseudomonas aeruginosa outer membrane porin protein F by using monoclonal antibodies. Infect Immun. 1983 Dec;42(3):1027–1033. doi: 10.1128/iai.42.3.1027-1033.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Menzel J., Golecki J. R., Speth V. Outer membrane of salmonella. Sites of export of newly synthesised lipopolysaccharide on the bacterial surface. Eur J Biochem. 1973 Jun 15;35(3):471–481. doi: 10.1111/j.1432-1033.1973.tb02861.x. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Sadoff J. C., Wright D. C., Futrovsky S., Sidberry H., Collins H., Kaufmann B. Characterization of mouse monoclonal antibodies directed against Pseudomonas aeruginosa lipopolysaccharides. Antibiot Chemother (1971) 1985;36:134–146. doi: 10.1159/000410478. [DOI] [PubMed] [Google Scholar]
- Shands J. W. Localization of Somatic Antigen on Gram-Negative Bacteria by Electron Microscopy. J Bacteriol. 1965 Jul;90(1):266–270. doi: 10.1128/jb.90.1.266-270.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Nikaido H. Outer membrane of gram-negative bacteria. XVIII. Electron microscopic studies on porin insertion sites and growth of cell surface of Salmonella typhimurium. J Bacteriol. 1978 Aug;135(2):687–702. doi: 10.1128/jb.135.2.687-702.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Whitfield C., Vimr E. R., Costerton J. W., Troy F. A. Protein synthesis is required for in vivo activation of polysialic acid capsule synthesis in Escherichia coli K1. J Bacteriol. 1984 Jul;159(1):321–328. doi: 10.1128/jb.159.1.321-328.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]