Abstract
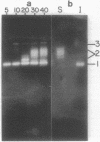
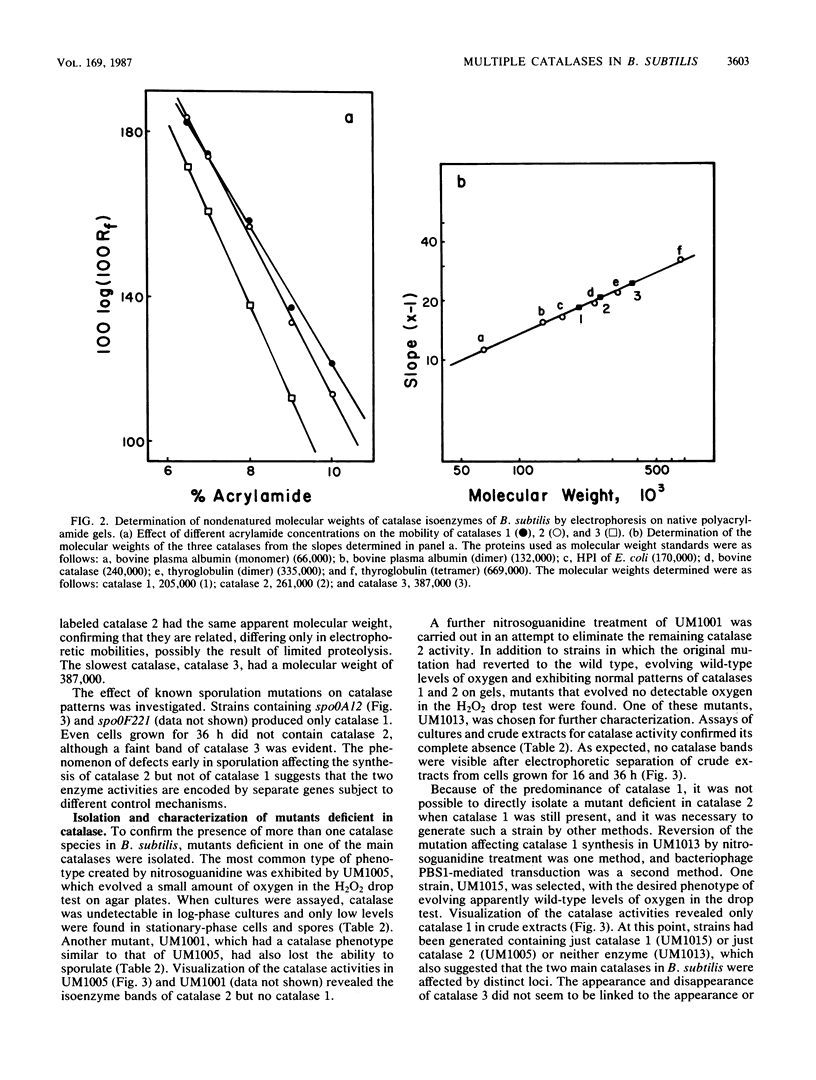
Vegetative cells of Bacillus subtilis in logarithmic growth phase produced one catalase, labeled catalase 1, with a nondenatured molecular weight of 205,000. As growth progressed, other activity bands with slower electrophoretic mobilities on polyacrylamide gels appeared, including a series of bands with a common nondenatured molecular weight of 261,000, collectively labeled catalase 2, and a minor band, with a molecular weight of 387,000, labeled catalase 3. Purified spores contained only catalase 2, and it was not produced in spo0A- or spo0F-containing mutants. Strains deficient in catalase 1 or catalase 2 or both were selected after mutagenesis. Sensitivities of the two main catalases to NaCN, NaN3, hydroxylamine, and temperature were similar, but the apparent Kms for H2O2 differed, being 36.6 and 64.4 mM, respectively, for catalase 1 and catalase 2. The levels of catalase 1 increased 15-fold during growth into stationary phase and could be increased 30-fold by the addition of H2O2 to the medium. Catalase 2, which was not affected by H2O2, appeared only after the cells had reached stationary phase, and the maximum levels were only half of the basal level of catalase 1.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Claiborne A., Fridovich I. Purification of the o-dianisidine peroxidase from Escherichia coli B. Physicochemical characterization and analysis of its dual catalatic and peroxidatic activities. J Biol Chem. 1979 May 25;254(10):4245–4252. [PubMed] [Google Scholar]
- Claiborne A., Malinowski D. P., Fridovich I. Purification and characterization of hydroperoxidase II of Escherichia coli B. J Biol Chem. 1979 Nov 25;254(22):11664–11668. [PubMed] [Google Scholar]
- Clare D. A., Duong M. N., Darr D., Archibald F., Fridovich I. Effects of molecular oxygen on detection of superoxide radical with nitroblue tetrazolium and on activity stains for catalase. Anal Biochem. 1984 Aug 1;140(2):532–537. doi: 10.1016/0003-2697(84)90204-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dingman D. W., Stahly D. P. Protection of Bacillus larvae from Oxygen Toxicity with Emphasis on the Role of Catalase. Appl Environ Microbiol. 1984 Jun;47(6):1228–1237. doi: 10.1128/aem.47.6.1228-1237.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Visualization of catalase on acrylamide gels. Anal Biochem. 1974 Mar;58(1):57–62. doi: 10.1016/0003-2697(74)90440-0. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Jacob G. S., Orme-Johnson W. H. Catalase of Neurospora crassa. 1. Induction, purification, and physical properties. Biochemistry. 1979 Jul 10;18(14):2967–2975. doi: 10.1021/bi00581a009. [DOI] [PubMed] [Google Scholar]
- Jouve H. M., Lasauniere C., Pelmont J. Properties of a catalase from a peroxide-resistant mutant of Proteus mirabilis. Can J Biochem Cell Biol. 1983 Nov;61(11):1219–1227. doi: 10.1139/o83-157. [DOI] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Loewen P. C., Switala J., Triggs-Raine B. L. Catalases HPI and HPII in Escherichia coli are induced independently. Arch Biochem Biophys. 1985 Nov 15;243(1):144–149. doi: 10.1016/0003-9861(85)90782-9. [DOI] [PubMed] [Google Scholar]
- Loewen P. C., Triggs B. L., George C. S., Hrabarchuk B. E. Genetic mapping of katG, a locus that affects synthesis of the bifunctional catalase-peroxidase hydroperoxidase I in Escherichia coli. J Bacteriol. 1985 May;162(2):661–667. doi: 10.1128/jb.162.2.661-667.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter H. E., Loewen P. C. Catalase synthesis in Escherichia coli is not controlled by catabolite repression. Arch Biochem Biophys. 1982 Apr 15;215(1):72–77. doi: 10.1016/0003-9861(82)90280-6. [DOI] [PubMed] [Google Scholar]
- Rorth M., Jensen P. K. Determination of catalase activity by means of the Clark oxygen electrode. Biochim Biophys Acta. 1967 May 16;139(1):171–173. doi: 10.1016/0005-2744(67)90124-6. [DOI] [PubMed] [Google Scholar]
- Scandalios J. G., Chang D. Y., McMillin D. E., Tsaftaris A., Moll R. H. Genetic regulation of the catalase developmental program in maize scutellum: Identification of a temporal regulatory gene. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5360–5364. doi: 10.1073/pnas.77.9.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seah T. C., Bhatti A. R., Kaplan J. G. Novel catalatic proteins of bakers' yeast. I. An atypical catalase. Can J Biochem. 1973 Nov;51(11):1551–1555. doi: 10.1139/o73-208. [DOI] [PubMed] [Google Scholar]
- Seah T. C., Kaplan J. G. Purification and properties of the catalase of bakers' yeast. J Biol Chem. 1973 Apr 25;248(8):2889–2893. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeyar M. A., Zahler S. A. Chromosomal insertions of Tn917 in Bacillus subtilis. J Bacteriol. 1986 Aug;167(2):530–534. doi: 10.1128/jb.167.2.530-534.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilyadi M., Archibald F. Catalase, superoxide dismutase, and the production of O2-sensitive mutants of Bacillus coagulans. Can J Microbiol. 1985 Nov;31(11):994–999. doi: 10.1139/m85-188. [DOI] [PubMed] [Google Scholar]