Abstract
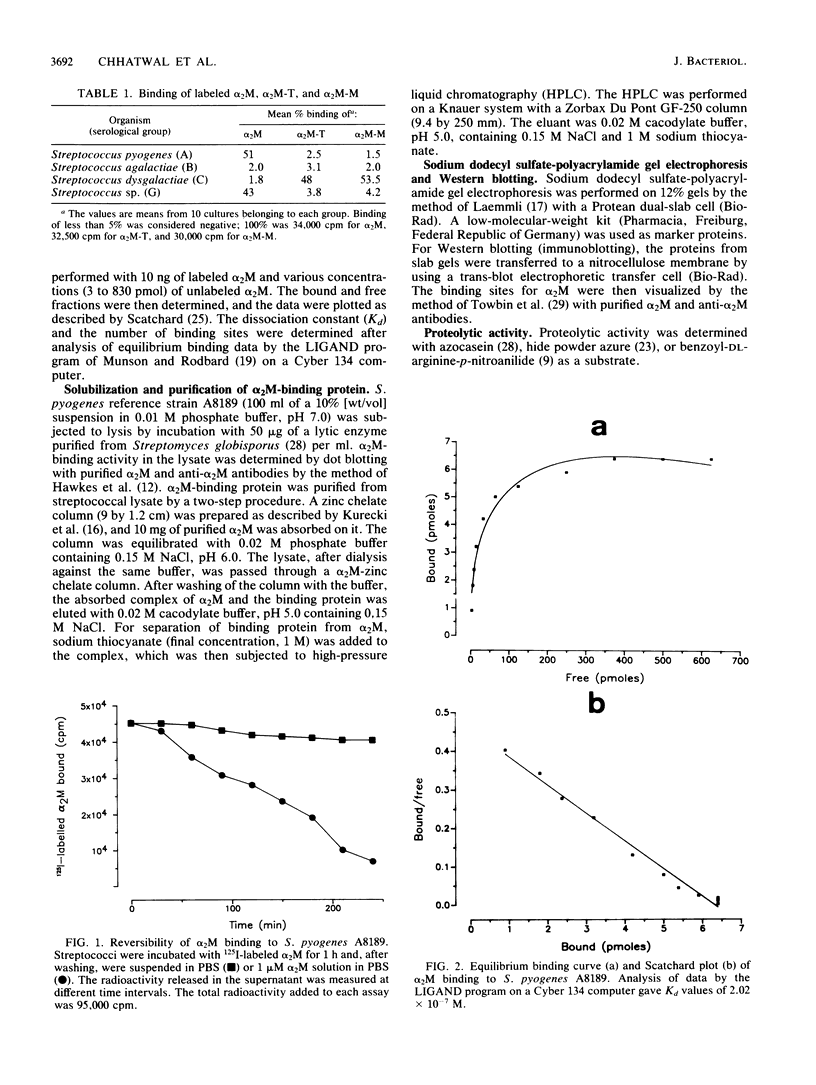
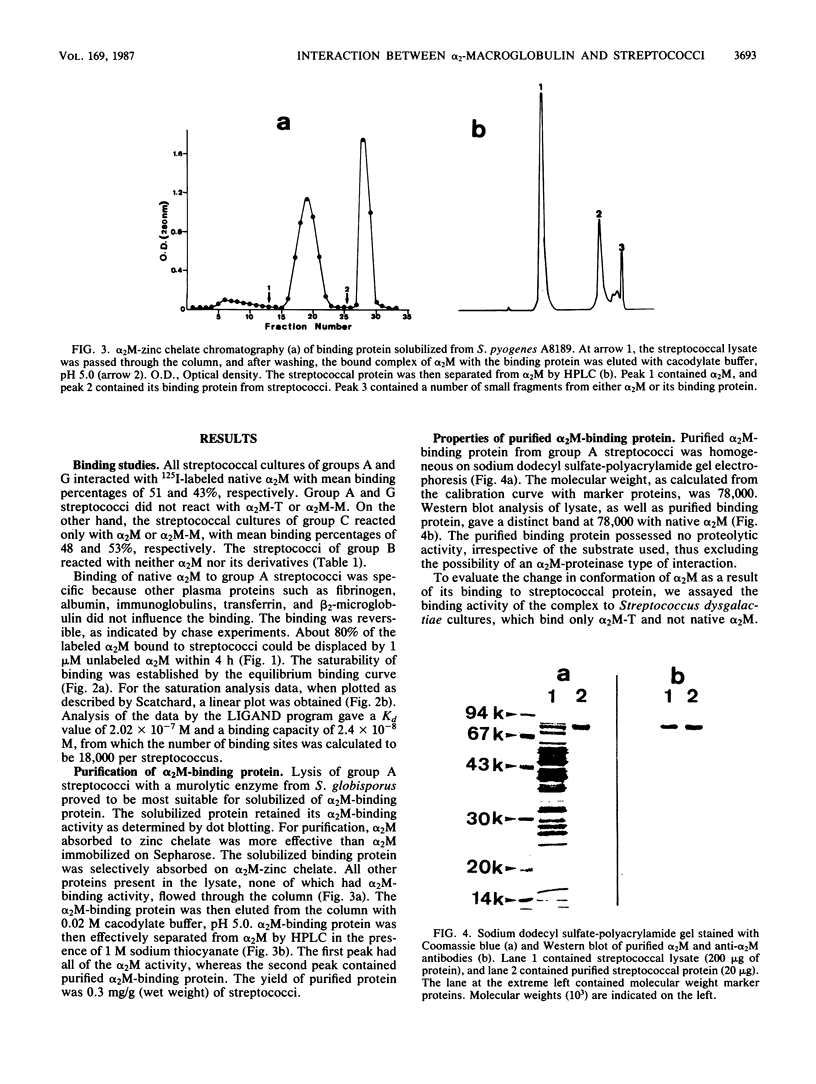
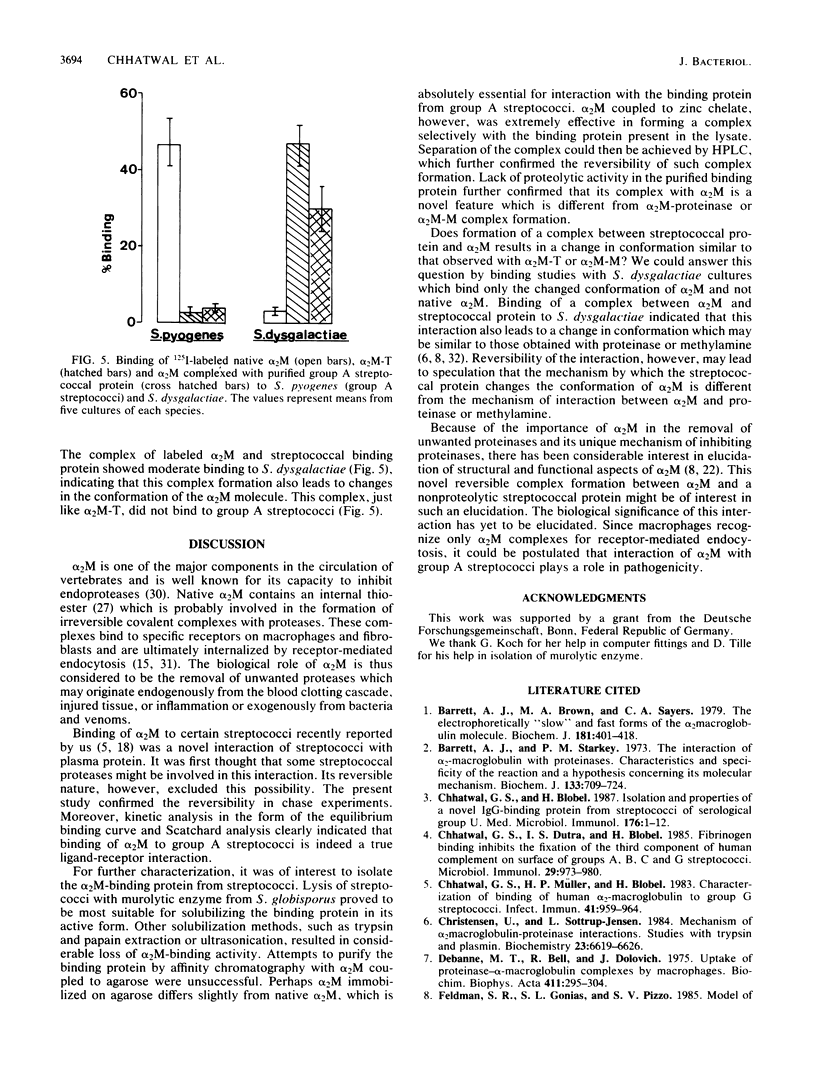
Binding of 125I-labeled alpha 2-macroglobulin (alpha 2M) to streptococci belonging to serological groups A, B, C, and G was studied. Streptococci of groups A and G interacted only with native alpha 2M, and those of group C reacted only with alpha 2M-trypsin complex. Binding of alpha 2M to group A streptococci was saturable and reversible. The dissociation constant was 2.02 X 10(-7) M, and the number of binding sites was calculated to be 18,000 per streptococcus. The alpha 2M-binding protein could be solubilized by treatment of group A streptococci with a murolytic enzyme and subsequently purified by affinity chromatography and high-pressure liquid chromatography. The purified protein was homogeneous on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and had a molecular weight of 78,000. It possessed no proteolytic activity and interacted with native alpha 2M in Western blots (immunoblots). Interaction of purified binding protein with alpha 2M led to a change in the conformation of alpha 2M similar to that obtained by alpha 2M-protease complexes. Reversible binding of a nonproteolytic streptococcal component of alpha 2M is thus a novel feature of alpha 2M reactivity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal G. S., Blobel H. Isolation and properties of a novel IgG-binding protein from streptococci of serological group U. Med Microbiol Immunol. 1987;176(1):1–12. doi: 10.1007/BF00189403. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Dutra I. S., Blobel H. Fibrinogen binding inhibits the fixation of the third component of human complement on surface of groups A, B, C, and G streptococci. Microbiol Immunol. 1985;29(10):973–980. doi: 10.1111/j.1348-0421.1985.tb02961.x. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Müller H. P., Blobel H. Characterization of binding of human alpha 2-macroglobulin to group G streptococci. Infect Immun. 1983 Sep;41(3):959–964. doi: 10.1128/iai.41.3.959-964.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen U., Sottrup-Jensen L. Mechanism of alpha 2-macroglobulin-proteinase interactions. Studies with trypsin and plasmin. Biochemistry. 1984 Dec 18;23(26):6619–6626. doi: 10.1021/bi00321a052. [DOI] [PubMed] [Google Scholar]
- Debanne M. T., Bell R., Dolovich J. Uptake of proteinase-alpha-macroglobulin complexes by macrophages. Biochim Biophys Acta. 1975 Dec 5;411(2):295–304. doi: 10.1016/0304-4165(75)90309-8. [DOI] [PubMed] [Google Scholar]
- Feldman S. R., Gonias S. L., Pizzo S. V. Model of alpha 2-macroglobulin structure and function. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5700–5704. doi: 10.1073/pnas.82.17.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganrot P. O. Determination of alpha-2-macroglobulin as trypsin-protein esterase. Clin Chim Acta. 1966 Oct;14(4):493–501. doi: 10.1016/0009-8981(66)90037-4. [DOI] [PubMed] [Google Scholar]
- Gonias S. L., Reynolds J. A., Pizzo S. V. Physical properties of human alpha 2-macroglobulin following reaction with methylamine and trypsin. Biochim Biophys Acta. 1982 Aug 10;705(3):306–314. doi: 10.1016/0167-4838(82)90252-7. [DOI] [PubMed] [Google Scholar]
- Hall P. K., Roberts R. C. Physical and chemical properties of human plasma alpha2-macroglobulin. Biochem J. 1978 Jul 1;173(1):27–38. doi: 10.1042/bj1730027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V. Clearance and binding of two electrophoretic "fast" forms of human alpha 2-macroglobulin. J Biol Chem. 1981 Aug 10;256(15):8134–8139. [PubMed] [Google Scholar]
- Kaplan J., Nielsen M. L. Analysis of macrophage surface receptors. I. Binding of alpha-macroglobulin . protease complexes to rabbit alveolar macrophages. J Biol Chem. 1979 Aug 10;254(15):7323–7328. [PubMed] [Google Scholar]
- Kaplan J., Nielsen M. L. Analysis of macrophage surface receptors. II. Internalization of alpha-macroglobulin . trypsin complexes by rabbit alveolar macrophages. J Biol Chem. 1979 Aug 10;254(15):7329–7335. [PubMed] [Google Scholar]
- Kurecki T., Kress L. F., Laskowski M., Sr Purification of human plasma alpha 2 macroglobulin and alpha 1 proteinase inhibitor using zinc chelate chromatography. Anal Biochem. 1979 Nov 1;99(2):415–420. doi: 10.1016/s0003-2697(79)80026-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Ney K. A., Gidwitz S., Pizzo S. V. Changes in the binding of "fast"-form alpha 2-macroglobulin to 3T3-L1 cells after differentiation to adipocytes. Biochemistry. 1984 Jul 17;23(15):3395–3403. doi: 10.1021/bi00310a003. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Skude G. Demonstration and semiquantitative determination of complexes between various proteases and human alpha2-macroglobulin. Clin Chim Acta. 1976 Jan 2;66(1):1–7. doi: 10.1016/0009-8981(76)90365-x. [DOI] [PubMed] [Google Scholar]
- Osterberg R., Malmensten B. Methylamine-induced conformational change of alpha 2-macroglobulin and its zinc (II) binding capacity. An X-ray scattering study. Eur J Biochem. 1984 Sep 17;143(3):541–544. doi: 10.1111/j.1432-1033.1984.tb08403.x. [DOI] [PubMed] [Google Scholar]
- Salvesen G. S., Sayers C. A., Barrett A. J. Further characterization of the covalent linking reaction of alpha 2-macroglobulin. Biochem J. 1981 May 1;195(2):453–461. doi: 10.1042/bj1950453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Amino acid sequence of the tryptic peptide containing the alkylamine-reactive site from human alpha 2-macroglobulin. Identification of gamma-glutamylmethylamide. J Biol Chem. 1980 Sep 10;255(17):8087–8091. [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Characterization of alkylamine-sensitive site in alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4313–4316. doi: 10.1073/pnas.76.9.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tille D., Chhatwal G. S., Blobel H. Release of Fc-receptors after streptococcal lysis induced by a lytic enzyme from Streptomyces globisporus. Med Microbiol Immunol. 1986;175(1):35–41. doi: 10.1007/BF02123127. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van Den Berghe H. Demonstration of an alpha2-macroglobulin receptor in human fibroblasts, absent in tumor-derived cell lines. J Biol Chem. 1979 Jun 25;254(12):5155–5160. [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van den Berghe H. Functional modifications of alpha 2-macroglobulin by primary amines. Kinetics of inactivation of alpha 2-macroglobulin by methylamine, and formation of anomalous complexes with trypsin. Biochem J. 1982 Jan 1;201(1):119–128. doi: 10.1042/bj2010119. [DOI] [PMC free article] [PubMed] [Google Scholar]