Abstract
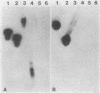
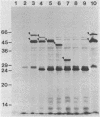
A 2.7-kilobase Sau3A fragment of Spirochaeta aurantia DNA cloned in pBR322 complemented a trpE deletion in Escherichia coli. Deletion analysis and Tn5 mutagenesis of the resulting plasmid pBG100 defined a 2-kilobase-pair region that was required for both the complementation and the synthesis of 59,000- and 47,000-molecular-weight polypeptides (59K and 47K polypeptides) in maxicells. Both the 59K and the 47K polypeptides appear to be encoded by a single gene. A maxicell analysis of pBG100::Tn5 mutants suggests that the 47K polypeptide is not sufficient for the trpE complementation. In vitro and in vivo anthranilate synthetase (AS) assays indicate that the complementing activity encoded by pBG100 was functionally analogous to the AS component I of E. coli in that it utilized NH3 but not glutamine as the amino donor. pBG100 did not encode a glutamine amidotransferase activity, although the AS component I it encoded was capable of interacting with E. coli AS component II to catalyze the glutamine-requiring reaction. Expression appeared to depend on a promoter in the cloned S. aurantia DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band L., Shimotsu H., Henner D. J. Nucleotide sequence of the Bacillus subtilis trpE and trpD genes. Gene. 1984 Jan;27(1):55–65. doi: 10.1016/0378-1119(84)90238-5. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Breznak J. A., Canale-Parola E. Morphology and physiology of Spirochaeta aurantia strains isolated from aquatic habitats. Arch Microbiol. 1975 Sep 30;105(1):1–12. doi: 10.1007/BF00447104. [DOI] [PubMed] [Google Scholar]
- Charnetzky W. T., Mortlock R. P. Ribitol catabolic pathway in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):162–169. doi: 10.1128/jb.119.1.162-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton J. L., Zamir A., Szalay A. A. Insertion of nonhomologous DNA into the yeast genome mediated by homologous recombination with a cotransforming plasmid. Mol Gen Genet. 1982;188(1):44–50. doi: 10.1007/BF00332994. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Comparative studies on the regulation of tryptophan synthesis. CRC Crit Rev Biochem. 1980;8(2):175–189. doi: 10.3109/10409238009105468. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Eberly L. Structure and regulation of the anthranilate synthase genes in Pseudomonas aeruginosa: I. Sequence of trpG encoding the glutamine amidotransferase subunit. Mol Biol Evol. 1986 Sep;3(5):436–448. doi: 10.1093/oxfordjournals.molbev.a040408. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Gene rearrangements in the evolution of the tryptophan synthetic pathway. Bacteriol Rev. 1975 Jun;39(2):87–120. doi: 10.1128/br.39.2.87-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Wilde A., Yelverton E. M., Figurski D., Hedges R. W. Structure and regulation of the anthranilate synthase genes in Pseudomonas aeruginosa: II. Cloning and expression in Escherichia coli. Mol Biol Evol. 1986 Sep;3(5):449–458. doi: 10.1093/oxfordjournals.molbev.a040409. [DOI] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Chemotaxis in Spirochaeta aurantia. J Bacteriol. 1977 Apr;130(1):485–494. doi: 10.1128/jb.130.1.485-494.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Localization of two functions of the phosphoribosyl anthranilate transferase of Escherichia coli to distinct regions of the polypeptide chain. J Bacteriol. 1974 Feb;117(2):502–508. doi: 10.1128/jb.117.2.502-508.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nichols B. P., Miozzari G. F., van Cleemput M., Bennett G. N., Yanofsky C. Nucleotide sequences of the trpG regions of Escherichia coli, Shigella dysenteriae, Salmonella typhimurium and Serratia marcescens. J Mol Biol. 1980 Oct 5;142(4):503–517. doi: 10.1016/0022-2836(80)90260-0. [DOI] [PubMed] [Google Scholar]
- Nichols B. P., van Cleemput M., Yanofsky C. Nucleotide sequence of Escherichia coli trpE. Anthranilate synthetase component I contains no tryptophan residues. J Mol Biol. 1981 Feb 15;146(1):45–54. doi: 10.1016/0022-2836(81)90365-x. [DOI] [PubMed] [Google Scholar]
- Queener S. F., Gunsalus I. C. Anthranilate synthase enzyme system and complementation in Pseudomonas species. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1225–1232. doi: 10.1073/pnas.67.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weiner A. M., Weber K. Natural read-through at the UGA termination signal of Q-beta coat protein cistron. Nat New Biol. 1971 Sep 15;234(50):206–209. doi: 10.1038/newbio234206a0. [DOI] [PubMed] [Google Scholar]
- Winkler M. E., Schoenlein P. V., Ross C. M., Barrett J. T., Ely B. Genetic and physical analyses of Caulobacter crescentus trp genes. J Bacteriol. 1984 Oct;160(1):279–287. doi: 10.1128/jb.160.1.279-287.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamao F., Muto A., Kawauchi Y., Iwami M., Iwagami S., Azumi Y., Osawa S. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Comparison of regulatory and structural regions of genes of tryptophan metabolism. Mol Biol Evol. 1984 Feb;1(2):143–161. doi: 10.1093/oxfordjournals.molbev.a040307. [DOI] [PubMed] [Google Scholar]
- Yelton D. B., Charon N. W. Cloning of a gene required for tryptophan biosynthesis from Leptospira biflexa serovar patoc into Escherichia coli. Gene. 1984 May;28(2):147–152. doi: 10.1016/0378-1119(84)90251-8. [DOI] [PubMed] [Google Scholar]
- Yelton D. B., Cohen R. A. Analysis of cloned DNA from Leptospira biflexa serovar patoc which complements a deletion of the Escherichia coli trpE gene. J Bacteriol. 1986 Jan;165(1):41–46. doi: 10.1128/jb.165.1.41-46.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin H., Kling D. Anthranilate synthetase. Purification and properties of component I from Salmonella typhimurium. Biochemistry. 1968 Oct;7(10):3566–3573. doi: 10.1021/bi00850a034. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Murphy T. Utilization of ammonia for tryptophan synthesis. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1370–1377. doi: 10.1016/0006-291x(75)90178-3. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]