Abstract
An integrative transformation system was established for an asporogenous methylotrophic yeast, Candida boidinii. This system uses a uracil auxotrophic mutant of C. boidinii as the host strain in combination with its URA3 gene as the selectable marker. First, the C. boidinii URA3 gene coding for orotidine-5'-phosphate decarboxylase (ODCase) was cloned by using complementation of the pyrF mutation of Escherichia coli. Next, the host ODCase-negative mutant strains (ura3 strains) were isolated by mutagenesis and selection for 5-fluro-orotic acid (5-FOA) resistance. Five ura3 host strains that exhibited both a low reversion rate and good methylotrophic growth were obtained. All of these strains could be transformed to Ura+ phenotype with a C. boidinii URA3-harboring plasmid linearized within the Candida DNA. The transformants had a stable Ura+ phenotype after nonselective growth for 10 generations. These results and extensive Southern analysis indicated that the linearized plasmid was integrated into the host chromosomal DNA by homologous recombination at the URA3 locus in C. boidinii.
Full text
PDF

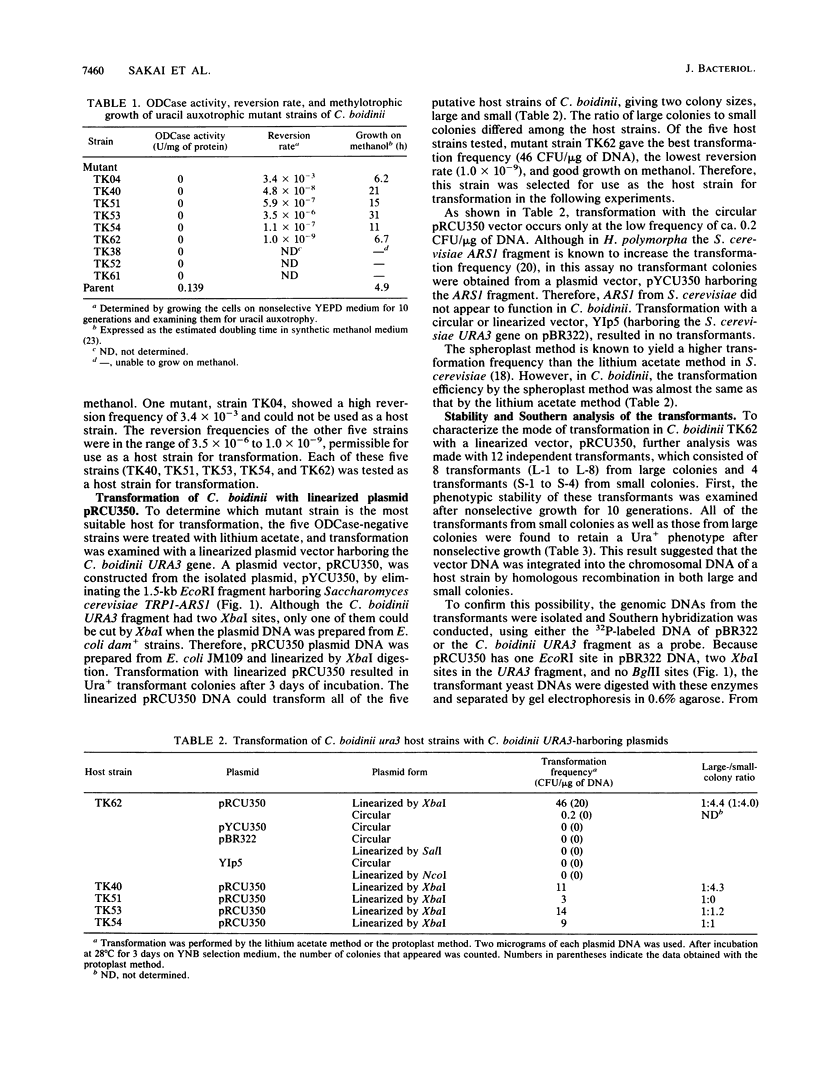
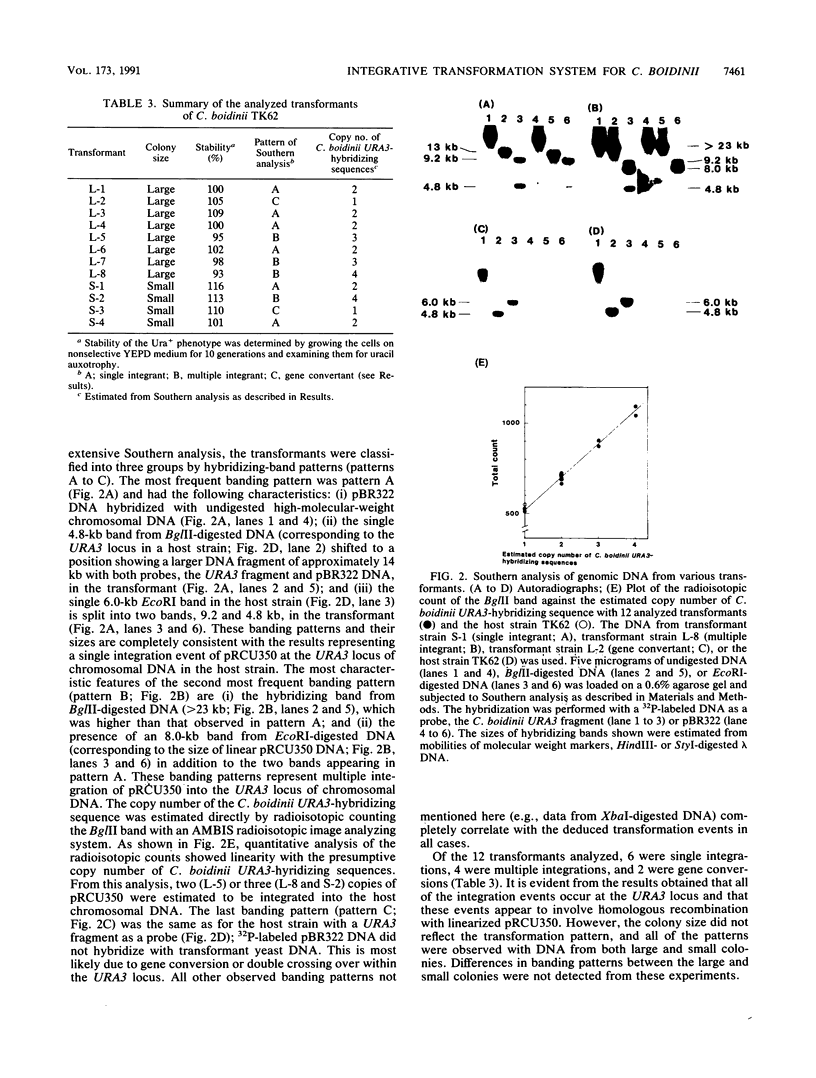


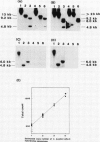
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alani E., Cao L., Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987 Aug;116(4):541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion E., Goodman J. M. Proton ionophores prevent assembly of a peroxisomal protein. Cell. 1987 Jan 16;48(1):165–173. doi: 10.1016/0092-8674(87)90367-9. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cregg J. M., Barringer K. J., Hessler A. Y., Madden K. R. Pichia pastoris as a host system for transformations. Mol Cell Biol. 1985 Dec;5(12):3376–3385. doi: 10.1128/mcb.5.12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer D. R., Eccleshall R., Marmur J. Isolation of yeast DNA. Methods Cell Biol. 1975;12:39–44. doi: 10.1016/s0091-679x(08)60950-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garrard L. J., Goodman J. M. Two genes encode the major membrane-associated protein of methanol-induced peroxisomes from Candida boidinii. J Biol Chem. 1989 Aug 15;264(23):13929–13937. [PubMed] [Google Scholar]
- Gleeson M. A., Haas L. O., Cregg J. M. Isolation of Candida tropicalis auxotrophic mutants. Appl Environ Microbiol. 1990 Aug;56(8):2562–2564. doi: 10.1128/aem.56.8.2562-2564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. M., Scott C. W., Donahue P. N., Atherton J. P. Alcohol oxidase assembles post-translationally into the peroxisome of Candida boidinii. J Biol Chem. 1984 Jul 10;259(13):8485–8493. [PubMed] [Google Scholar]
- Haas L. O., Cregg J. M., Gleeson M. A. Development of an integrative DNA transformation system for the yeast Candida tropicalis. J Bacteriol. 1990 Aug;172(8):4571–4577. doi: 10.1128/jb.172.8.4571-4577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R., Miller S. M., Kurtz M. B., Kirsch D. R. Directed mutagenesis in Candida albicans: one-step gene disruption to isolate ura3 mutants. Mol Cell Biol. 1987 Jan;7(1):199–208. doi: 10.1128/mcb.7.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M., Grisafi P., Botstein D. Structure and function of the yeast URA3 gene: expression in Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):113–124. doi: 10.1016/0378-1119(84)90172-0. [DOI] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Sawai T., Tani Y. Isolation and Characterization of a Catabolite Repression-Insensitive Mutant of a Methanol Yeast, Candida boidinii A5, Producing Alcohol Oxidase in Glucose-Containing Medium. Appl Environ Microbiol. 1987 Aug;53(8):1812–1818. doi: 10.1128/aem.53.8.1812-1818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Tani Y. Production of Formaldehyde by Detergent-Treated Cells of a Methanol Yeast, Candida boidinii S2 Mutant Strain AOU-1. Appl Environ Microbiol. 1988 Feb;54(2):485–489. doi: 10.1128/aem.54.2.485-489.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto A., Umezu K., Kobayashi K., Tomita K. Orotidylate decarboxylase (yeast). Methods Enzymol. 1978;51:74–79. doi: 10.1016/s0076-6879(78)51013-6. [DOI] [PubMed] [Google Scholar]