Abstract
We have isolated mutations in rpoA, the gene encoding the alpha subunit of RNA polymerase, that specifically affect transcriptional control by OmpR and EnvZ, the two-component regulatory system that controls porin gene expression in Escherichia coli. Characterization of these mutations and a previously isolated rpoA allele suggests that both positive and negative regulation of porin gene transcription involves a direct interaction between OmpR and RNA polymerase through the alpha subunit. Several of the rpoA mutations cluster in the carboxy-terminal portion of the alpha protein, further suggesting that it is this domain of alpha that is involved in interaction with OmpR and perhaps other transcriptional regulators as well.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Garges S. Positive control. J Biol Chem. 1990 Jul 5;265(19):10797–10800. [PubMed] [Google Scholar]
- Aiba H., Nakasai F., Mizushima S., Mizuno T. Evidence for the physiological importance of the phosphotransfer between the two regulatory components, EnvZ and OmpR, in osmoregulation in Escherichia coli. J Biol Chem. 1989 Aug 25;264(24):14090–14094. [PubMed] [Google Scholar]
- Bedwell D. M., Nomura M. Feedback regulation of RNA polymerase subunit synthesis after the conditional overproduction of RNA polymerase in Escherichia coli. Mol Gen Genet. 1986 Jul;204(1):17–23. doi: 10.1007/BF00330181. [DOI] [PubMed] [Google Scholar]
- Berman M. L., Jackson D. E. Selection of lac gene fusions in vivo: ompR-lacZ fusions that define a functional domain of the ompR gene product. J Bacteriol. 1984 Aug;159(2):750–756. doi: 10.1128/jb.159.2.750-756.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975 Aug 5;96(2):307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- Bushman F. D., Ptashne M. Turning lambda Cro into a transcriptional activator. Cell. 1988 Jul 15;54(2):191–197. doi: 10.1016/0092-8674(88)90551-x. [DOI] [PubMed] [Google Scholar]
- Bushman F. D., Shang C., Ptashne M. A single glutamic acid residue plays a key role in the transcriptional activation function of lambda repressor. Cell. 1989 Sep 22;58(6):1163–1171. doi: 10.1016/0092-8674(89)90514-x. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Case C. C., Bukau B., Granett S., Villarejo M. R., Boos W. Contrasting mechanisms of envZ control of mal and pho regulon genes in Escherichia coli. J Bacteriol. 1986 Jun;166(3):706–712. doi: 10.1128/jb.166.3.706-712.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie G. E., Haggård-Ljungquist E., Feiwell R., Calendar R. Regulation of bacteriophage P2 late-gene expression: the ogr gene. Proc Natl Acad Sci U S A. 1986 May;83(10):3238–3242. doi: 10.1073/pnas.83.10.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Delgado J., Rampersaud A., Inouye M. In vivo phosphorylation of OmpR, the transcription activator of the ompF and ompC genes in Escherichia coli. J Bacteriol. 1990 Jun;172(6):3473–3477. doi: 10.1128/jb.172.6.3473-3477.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett S., Silhavy T. J. Isolation of mutations in the alpha operon of Escherichia coli that suppress the transcriptional defect conferred by a mutation in the porin regulatory gene envZ. J Bacteriol. 1987 Apr;169(4):1379–1385. doi: 10.1128/jb.169.4.1379-1385.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
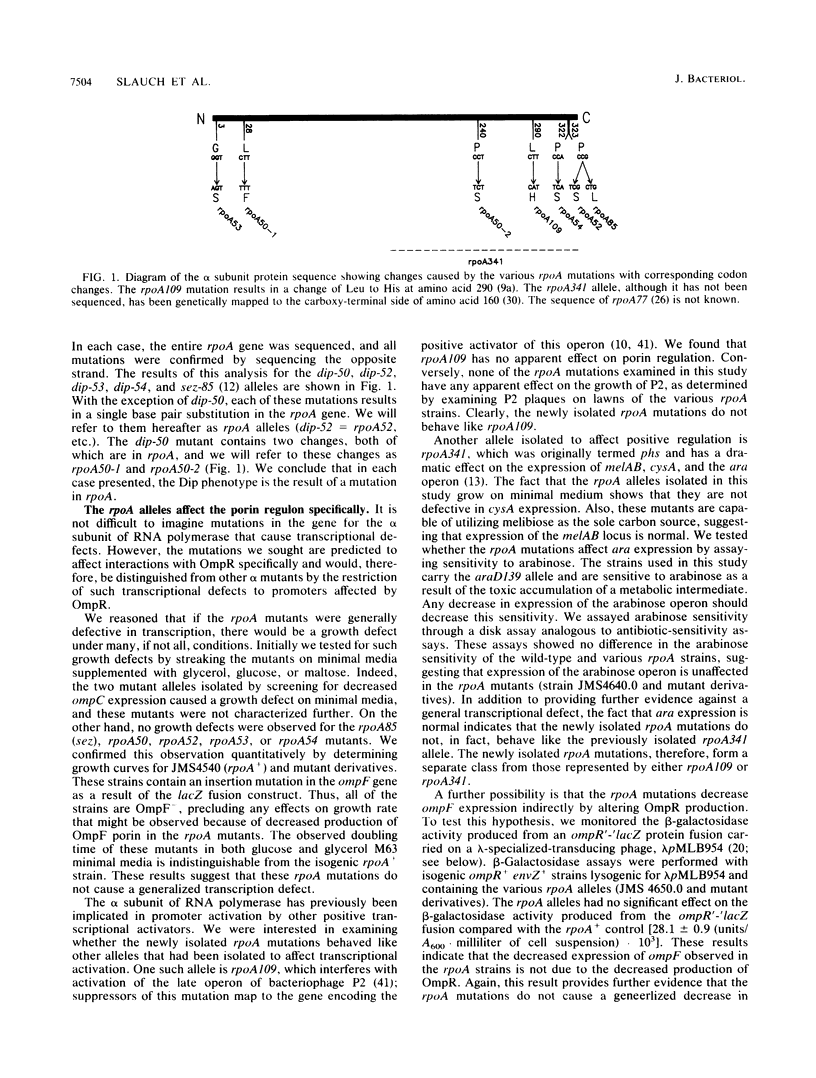
- Giffard P. M., Booth I. R. The rpoA341 allele of Escherichia coli specifically impairs the transcription of a group of positively-regulated operons. Mol Gen Genet. 1988 Sep;214(1):148–152. doi: 10.1007/BF00340193. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Transcriptional regulation of Escherichia coli K-12 major outer membrane protein 1b. J Bacteriol. 1979 Nov;140(2):342–350. doi: 10.1128/jb.140.2.342-350.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Bacteriophage resistance in Escherichia coli K-12: general pattern of resistance. J Bacteriol. 1975 Mar;121(3):983–993. doi: 10.1128/jb.121.3.983-993.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Mechanism of activation of transcription initiation from the lambda PRM promoter. J Mol Biol. 1982 May 25;157(3):493–525. doi: 10.1016/0022-2836(82)90473-9. [DOI] [PubMed] [Google Scholar]
- Hwang J. J., Gussin G. N. Interactions between Escherichia coli RNA polymerase and lambda repressor. Mutations in PRM affect repression of PR. J Mol Biol. 1988 Apr 20;200(4):735–739. doi: 10.1016/0022-2836(88)90484-6. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Ishihama A. Bipartite functional map of the E. coli RNA polymerase alpha subunit: involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell. 1991 Jun 14;65(6):1015–1022. doi: 10.1016/0092-8674(91)90553-b. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Slauch J. M., Silhavy T. J. Signal transduction in bacteria: kinases that control gene expression. New Biol. 1990 Jan;2(1):5–9. [PubMed] [Google Scholar]
- Kretz K. A., Carson G. S., O'Brien J. S. Direct sequencing from low-melt agarose with Sequenase. Nucleic Acids Res. 1989 Jul 25;17(14):5864–5864. doi: 10.1093/nar/17.14.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M. J., Bagga D., Miller C. G. Mutations in rpoA affect expression of anaerobically regulated genes in Salmonella typhimurium. J Bacteriol. 1991 Dec;173(23):7511–7518. doi: 10.1128/jb.173.23.7511-7518.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan T. P., Kolb A., Buc H., McClure W. R. Mechanism of CRP-cAMP activation of lac operon transcription initiation activation of the P1 promoter. J Mol Biol. 1984 Dec 25;180(4):881–909. doi: 10.1016/0022-2836(84)90262-6. [DOI] [PubMed] [Google Scholar]
- Martin K., Huo L., Schleif R. F. The DNA loop model for ara repression: AraC protein occupies the proposed loop sites in vivo and repression-negative mutations lie in these same sites. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3654–3658. doi: 10.1073/pnas.83.11.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Mizuno T., Mizushima S. Interaction between two regulatory proteins in osmoregulatory expression of ompF and ompC genes in Escherichia coli: a novel ompR mutation suppresses pleiotropic defects caused by an envZ mutation. J Bacteriol. 1986 Dec;168(3):1309–1314. doi: 10.1128/jb.168.3.1309-1314.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Mizushima S. Novel rpoA mutation that interferes with the function of OmpR and EnvZ, positive regulators of the ompF and ompC genes that code for outer-membrane proteins in Escherichia coli K12. J Mol Biol. 1987 Jun 20;195(4):847–853. doi: 10.1016/0022-2836(87)90489-x. [DOI] [PubMed] [Google Scholar]
- McCarthy J. E., Gualerzi C. Translational control of prokaryotic gene expression. Trends Genet. 1990 Mar;6(3):78–85. doi: 10.1016/0168-9525(90)90098-q. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow K. S., Silhavy T. J., Garrett S. cis-acting sites required for osmoregulation of ompF expression in Escherichia coli K-12. J Bacteriol. 1986 Dec;168(3):1165–1171. doi: 10.1128/jb.168.3.1165-1171.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland G. C., Giffard P. M., Booth I. R. phs Locus of Escherichia coli, a mutation causing pleiotropic lesions in metabolism, is an rpoA allele. J Bacteriol. 1985 Nov;164(2):972–975. doi: 10.1128/jb.164.2.972-975.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., McDonald G. A. Regulation of outer membrane protein synthesis in Escherichia coli K-12: deletion of ompC affects expression of the OmpF protein. J Bacteriol. 1984 Aug;159(2):555–563. doi: 10.1128/jb.159.2.555-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih M. C., Gussin G. N. Differential effects of mutations on discrete steps in transcription initiation at the lambda PRE promoter. Cell. 1983 Oct;34(3):941–949. doi: 10.1016/0092-8674(83)90551-2. [DOI] [PubMed] [Google Scholar]
- Slauch J. M., Garrett S., Jackson D. E., Silhavy T. J. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol. 1988 Jan;170(1):439–441. doi: 10.1128/jb.170.1.439-441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauch J. M., Silhavy T. J. Genetic analysis of the switch that controls porin gene expression in Escherichia coli K-12. J Mol Biol. 1989 Nov 20;210(2):281–292. doi: 10.1016/0022-2836(89)90330-6. [DOI] [PubMed] [Google Scholar]
- Slauch J. M., Silhavy T. J. cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J Bacteriol. 1991 Jul;173(13):4039–4048. doi: 10.1128/jb.173.13.4039-4048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straney S. B., Crothers D. M. Lac repressor is a transient gene-activating protein. Cell. 1987 Dec 4;51(5):699–707. doi: 10.1016/0092-8674(87)90093-6. [DOI] [PubMed] [Google Scholar]
- Su W., Porter S., Kustu S., Echols H. DNA-looping and enhancer activity: association between DNA-bound NtrC activator and RNA polymerase at the bacterial glnA promoter. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5504–5508. doi: 10.1073/pnas.87.14.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine M. G., Sauer B. A bacterial mutation blocking P2 phage late gene expression. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2770–2774. doi: 10.1073/pnas.72.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S., Richmond T. J. DNA binding-induced conformational change of the yeast transcriptional activator PRTF. Cell. 1990 Jul 27;62(2):367–377. doi: 10.1016/0092-8674(90)90373-m. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Garrett S., Sodergren E., Silhavy T. J. Mutations that define the promoter of ompF, a gene specifying a major outer membrane porin protein. J Bacteriol. 1985 Jun;162(3):1054–1060. doi: 10.1128/jb.162.3.1054-1060.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Moreno F., Schwartz M. Pleiotropic mutations rendering Escherichia coli K-12 resistant to bacteriophage TP1. J Bacteriol. 1980 Sep;143(3):1374–1383. doi: 10.1128/jb.143.3.1374-1383.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


