Abstract
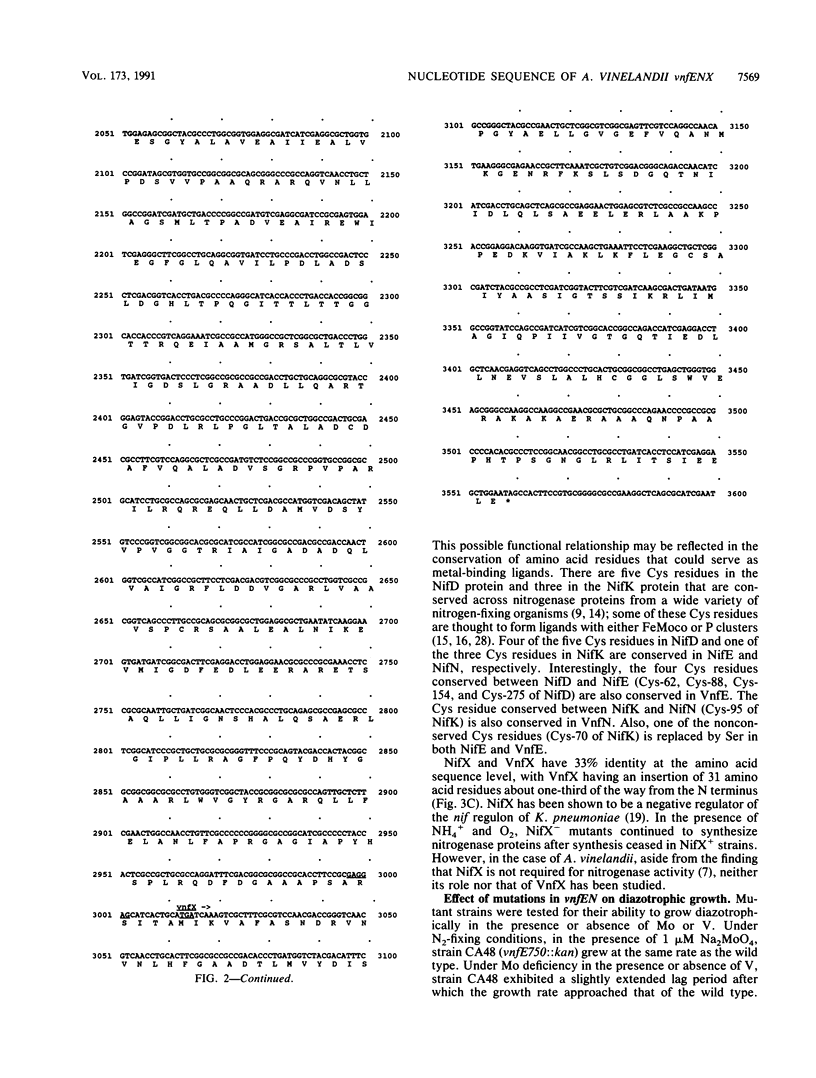
The nucleotide sequence (3,600 bp) of a second copy of nifENX-like genes in Azotobacter vinelandii has been determined. These genes are located immediately downstream from vnfA and have been designated vnfENX. The vnfENX genes appear to be organized as a single transcriptional unit that is preceded by a potential RpoN-dependent promoter. While the nifEN genes are thought to be evolutionarily related to nifDK, the vnfEN genes appear to be more closely related to nifEN than to either nifDK, vnfDK, or anfDK. Mutant strains (CA47 and CA48) carrying insertions in vnfE and vnfN, respectively, are able to grow diazotrophically in molybdenum (Mo)-deficient medium containing vanadium (V) (Vnf+) and in medium lacking both Mo and V (Anf+). However, a double mutant (strain DJ42.48) which contains a nifEN deletion and an insertion in vnfE is unable to grow diazotrophically in Mo-sufficient medium or in Mo-deficient medium with or without V. This suggests that NifE and NifN substitute for VnfE and VnfN when the vnfEN genes are mutationally inactivated. AnfA is not required for the expression of a vnfN-lacZ transcriptional fusion, even though this fusion is expressed under Mo- and V-deficient diazotrophic growth conditions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar O. M., Taormino J., Thöny B., Ramseier T., Hennecke H., Szalay A. A. The nifEN genes participating in FeMo cofactor biosynthesis and genes encoding dinitrogenase are part of the same operon in Bradyrhizobium species. Mol Gen Genet. 1990 Dec;224(3):413–420. doi: 10.1007/BF00262436. [DOI] [PubMed] [Google Scholar]
- Arnold W., Rump A., Klipp W., Priefer U. B., Pühler A. Nucleotide sequence of a 24,206-base-pair DNA fragment carrying the entire nitrogen fixation gene cluster of Klebsiella pneumoniae. J Mol Biol. 1988 Oct 5;203(3):715–738. doi: 10.1016/0022-2836(88)90205-7. [DOI] [PubMed] [Google Scholar]
- Beynon J., Cannon M., Buchanan-Wollaston V., Cannon F. The nif promoters of Klebsiella pneumoniae have a characteristic primary structure. Cell. 1983 Sep;34(2):665–671. doi: 10.1016/0092-8674(83)90399-9. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Hawkins M. E., Eady R. R. Nitrogen fixation in molybdenum-deficient continuous culture by a strain of Azotobacter vinelandii carrying a deletion of the structural genes for nitrogenase (nifHDK). Biochem J. 1986 Sep 1;238(2):437–442. doi: 10.1042/bj2380437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Premakumar R., Dean D. R., Jacobson M. R., Chisnell J. R., Rizzo T. M., Kopczynski J. Nitrogen Fixation by Azotobacter vinelandii Strains Having Deletions in Structural Genes for Nitrogenase. Science. 1986 Apr 4;232(4746):92–94. doi: 10.1126/science.232.4746.92. [DOI] [PubMed] [Google Scholar]
- Brigle K. E., Newton W. E., Dean D. R. Complete nucleotide sequence of the Azotobacter vinelandii nitrogenase structural gene cluster. Gene. 1985;37(1-3):37–44. doi: 10.1016/0378-1119(85)90255-0. [DOI] [PubMed] [Google Scholar]
- Brigle K. E., Weiss M. C., Newton W. E., Dean D. R. Products of the iron-molybdenum cofactor-specific biosynthetic genes, nifE and nifN, are structurally homologous to the products of the nitrogenase molybdenum-iron protein genes, nifD and nifK. J Bacteriol. 1987 Apr;169(4):1547–1553. doi: 10.1128/jb.169.4.1547-1553.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess B. K., Jacobs D. B., Stiefel E. I. Large-scale purification of high activity Azotobacter vinelandII nitrogenase. Biochim Biophys Acta. 1980 Jul 10;614(1):196–209. doi: 10.1016/0005-2744(80)90180-1. [DOI] [PubMed] [Google Scholar]
- Chisnell J. R., Premakumar R., Bishop P. E. Purification of a second alternative nitrogenase from a nifHDK deletion strain of Azotobacter vinelandii. J Bacteriol. 1988 Jan;170(1):27–33. doi: 10.1128/jb.170.1.27-33.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE WITT C. W., ROWE J. A. N,O-Diacetylneuraminic acid and N-acetylneuraminic acid in Escherichia coli. Nature. 1959 Aug 1;184(Suppl 6):381–382. doi: 10.1038/184381b0. [DOI] [PubMed] [Google Scholar]
- Dean D. R., Brigle K. E. Azotobacter vinelandii nifD- and nifE-encoded polypeptides share structural homology. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5720–5723. doi: 10.1073/pnas.82.17.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. R., Setterquist R. A., Brigle K. E., Scott D. J., Laird N. F., Newton W. E. Evidence that conserved residues Cys-62 and Cys-154 within the Azotobacter vinelandii nitrogenase MoFe protein alpha-subunit are essential for nitrogenase activity but conserved residues His-83 and Cys-88 are not. Mol Microbiol. 1990 Sep;4(9):1505–1512. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosink M. M., Franklin N. M., Roberts G. P. The product of the Klebsiella pneumoniae nifX gene is a negative regulator of the nitrogen fixation (nif) regulon. J Bacteriol. 1990 Mar;172(3):1441–1447. doi: 10.1128/jb.172.3.1441-1447.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales B. J., Langosch D. J., Case E. E. Isolation and characterization of a second nitrogenase Fe-protein from Azotobacter vinelandii. J Biol Chem. 1986 Nov 15;261(32):15301–15306. [PubMed] [Google Scholar]
- Jacobson M. R., Brigle K. E., Bennett L. T., Setterquist R. A., Wilson M. S., Cash V. L., Beynon J., Newton W. E., Dean D. R. Physical and genetic map of the major nif gene cluster from Azotobacter vinelandii. J Bacteriol. 1989 Feb;171(2):1017–1027. doi: 10.1128/jb.171.2.1017-1027.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Bishop P. E. Bacterial alternative nitrogen fixation systems. Crit Rev Microbiol. 1988;16(1):1–14. doi: 10.3109/10408418809104465. [DOI] [PubMed] [Google Scholar]
- Joerger R. D., Jacobson M. R., Bishop P. E. Two nifA-like genes required for expression of alternative nitrogenases by Azotobacter vinelandii. J Bacteriol. 1989 Jun;171(6):3258–3267. doi: 10.1128/jb.171.6.3258-3267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Jacobson M. R., Premakumar R., Wolfinger E. D., Bishop P. E. Nucleotide sequence and mutational analysis of the structural genes (anfHDGK) for the second alternative nitrogenase from Azotobacter vinelandii. J Bacteriol. 1989 Feb;171(2):1075–1086. doi: 10.1128/jb.171.2.1075-1086.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger R. D., Loveless T. M., Pau R. N., Mitchenall L. A., Simon B. H., Bishop P. E. Nucleotide sequences and mutational analysis of the structural genes for nitrogenase 2 of Azotobacter vinelandii. J Bacteriol. 1990 Jun;172(6):3400–3408. doi: 10.1128/jb.172.6.3400-3408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. E., Mackenzie J. M., Jr, Dougherty W. G. Assembly of overlapping DNA sequences by a program written in BASIC for 64K CP/M and MS-DOS IBM-compatible microcomputers. Nucleic Acids Res. 1986 Jan 10;14(1):517–527. doi: 10.1093/nar/14.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent H. M., Baines M., Gormal C., Smith B. E., Buck M. Analysis of site-directed mutations in the alpha- and beta-subunits of Klebsiella pneumoniae nitrogenase. Mol Microbiol. 1990 Sep;4(9):1497–1504. [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Vivian C., Schmehl M., Masepohl B., Arnold W., Klipp W. DNA sequence and genetic analysis of the Rhodobacter capsulatus nifENX gene region: homology between NifX and NifB suggests involvement of NifX in processing of the iron-molybdenum cofactor. Mol Gen Genet. 1989 Apr;216(2-3):353–363. doi: 10.1007/BF00334376. [DOI] [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Optimal conditions for transformation of Azotobacter vinelandii. J Bacteriol. 1979 Sep;139(3):1058–1061. doi: 10.1128/jb.139.3.1058-1061.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paustian T. D., Shah V. K., Roberts G. P. Apodinitrogenase: purification, association with a 20-kilodalton protein, and activation by the iron-molybdenum cofactor in the absence of dinitrogenase reductase. Biochemistry. 1990 Apr 10;29(14):3515–3522. doi: 10.1021/bi00466a014. [DOI] [PubMed] [Google Scholar]
- Paustian T. D., Shah V. K., Roberts G. P. Purification and characterization of the nifN and nifE gene products from Azotobacter vinelandii mutant UW45. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6082–6086. doi: 10.1073/pnas.86.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. C., Burgess B. K., Dean D. R. Activity, reconstitution, and accumulation of nitrogenase components in Azotobacter vinelandii mutant strains containing defined deletions within the nitrogenase structural gene cluster. J Bacteriol. 1986 Apr;166(1):180–186. doi: 10.1128/jb.166.1.180-186.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Woodley P. R., Pau R. N., Eady R. R. Structural genes for the vanadium nitrogenase from Azotobacter chroococcum. EMBO J. 1989 Apr;8(4):1217–1224. doi: 10.1002/j.1460-2075.1989.tb03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setterquist R., Brigle K. E., Beynon J., Cannon M., Ally A., Cannon F., Dean D. R. Nucleotide sequence of the nifE and nifN genes from Klebsiella pneumoniae. Nucleic Acids Res. 1988 Jun 10;16(11):5215–5215. doi: 10.1093/nar/16.11.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R., Quandt J., Klipp W. New derivatives of transposon Tn5 suitable for mobilization of replicons, generation of operon fusions and induction of genes in gram-negative bacteria. Gene. 1989 Aug 1;80(1):161–169. doi: 10.1016/0378-1119(89)90262-x. [DOI] [PubMed] [Google Scholar]
- Smith B. E., Eady R. R., Lowe D. J., Gormal C. The vanadium-iron protein of vanadium nitrogenase from Azotobacter chroococcum contains an iron-vanadium cofactor. Biochem J. 1988 Feb 15;250(1):299–302. doi: 10.1042/bj2500299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]
- Ugalde R. A., Imperial J., Shah V. K., Brill W. J. Biosynthesis of iron-molybdenum cofactor in the absence of nitrogenase. J Bacteriol. 1984 Sep;159(3):888–893. doi: 10.1128/jb.159.3.888-893.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


