Abstract
We previously observed (W. Godchaux, L. Gorski, and E.R. Leadbetter, J. Bacteriol. 172:1250-1255, 1990) that two mutants (strains 21 and NS-1) of the gliding bacterium Cytophaga johnsonae that were totally deficient in motility-dependent colony spreading, movement of rafts (groups) of cells as observed with a microscope, and movement of polystyrene-latex spheres that attached to the cell surface (observed in wet mounts) were also deficient in a high-molecular-weight cell surface polysaccharide (HMPS) and suggested a role for that substance in gliding motility. Antisera have been prepared against the purified HMPS, and these were used to select mutants specifically and highly deficient in the polysaccharide. All five such mutants had rates of colony spreading and raft movement that were much lower than those of the parent strain, but the rate of increase in colony diameter was higher than that found for strains NS-1 and 21 (which do not undergo raft movement at all). Unlike these latter two strains, the HMPS mutants retained the ability to move polystyrene-latex spheres over their surfaces. Hence, HMPS deficiency results in defective motility but not nonmotility, and the HMPS deficiency cannot fully explain the phenotype of mutants 21 and NS-1; in these strains, gliding must be affected by additional biochemical lesions. The HMPS may, nonetheless, be advantageous in that it supports greater gliding speeds.
Full text
PDF


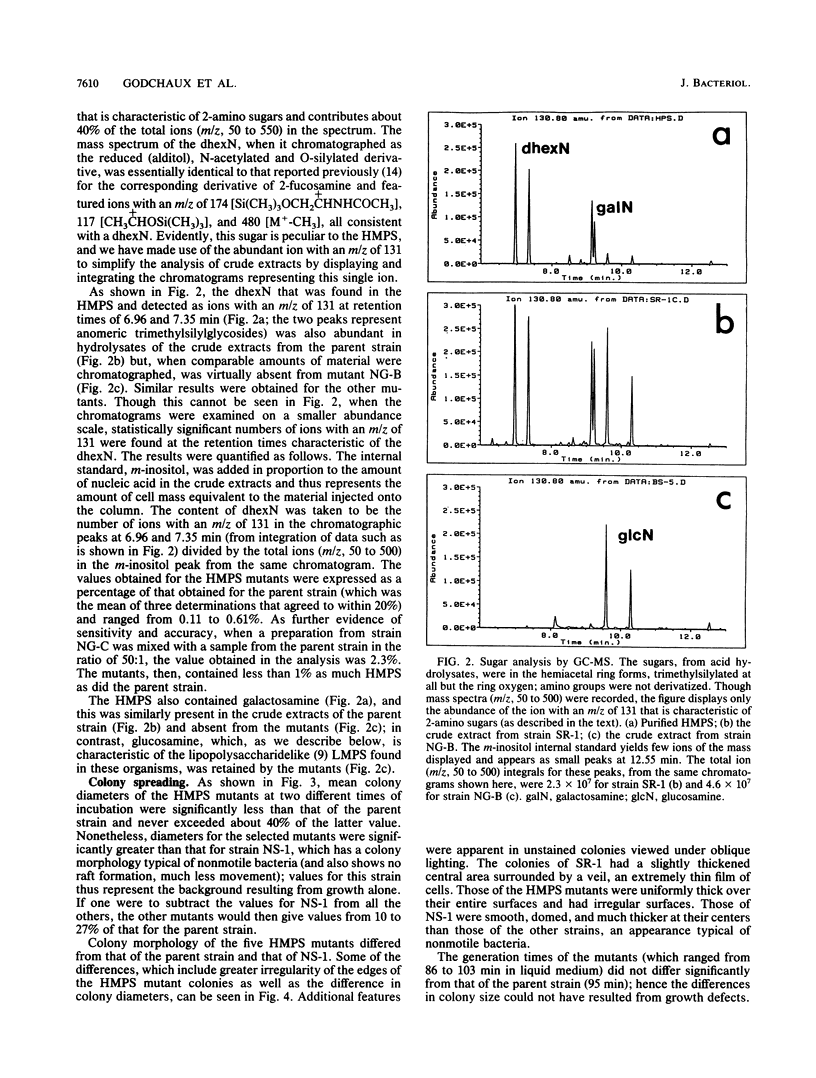
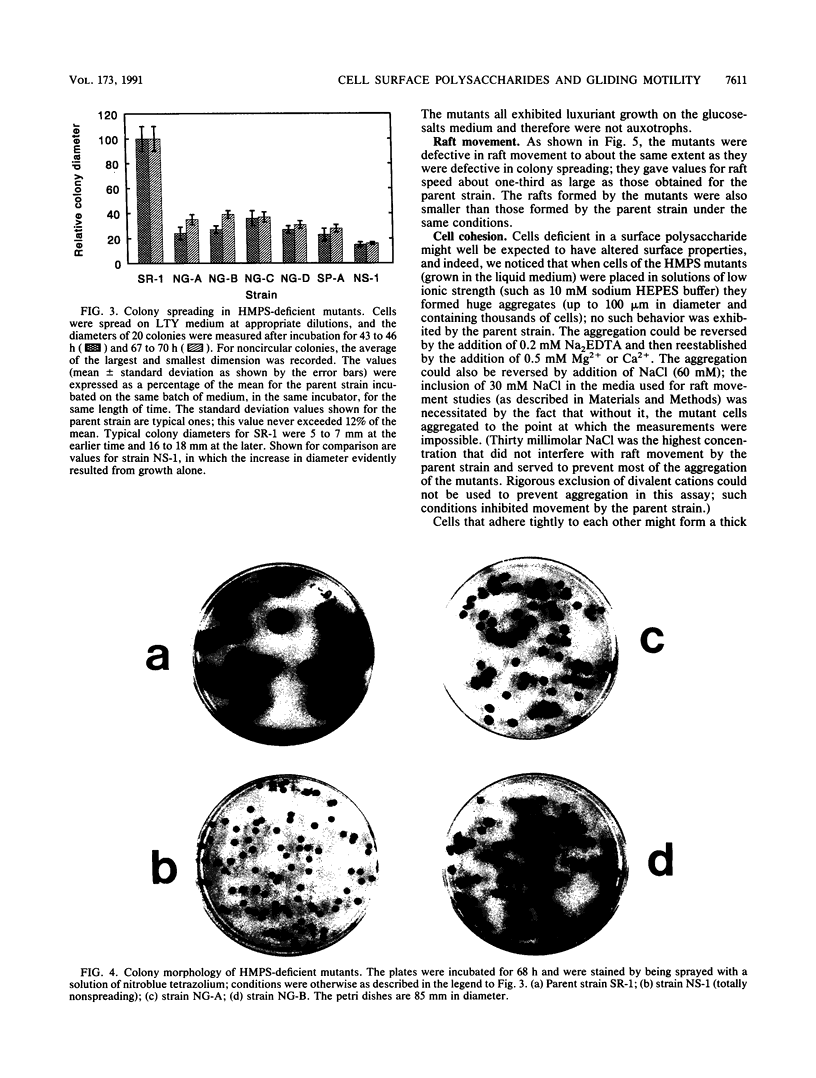
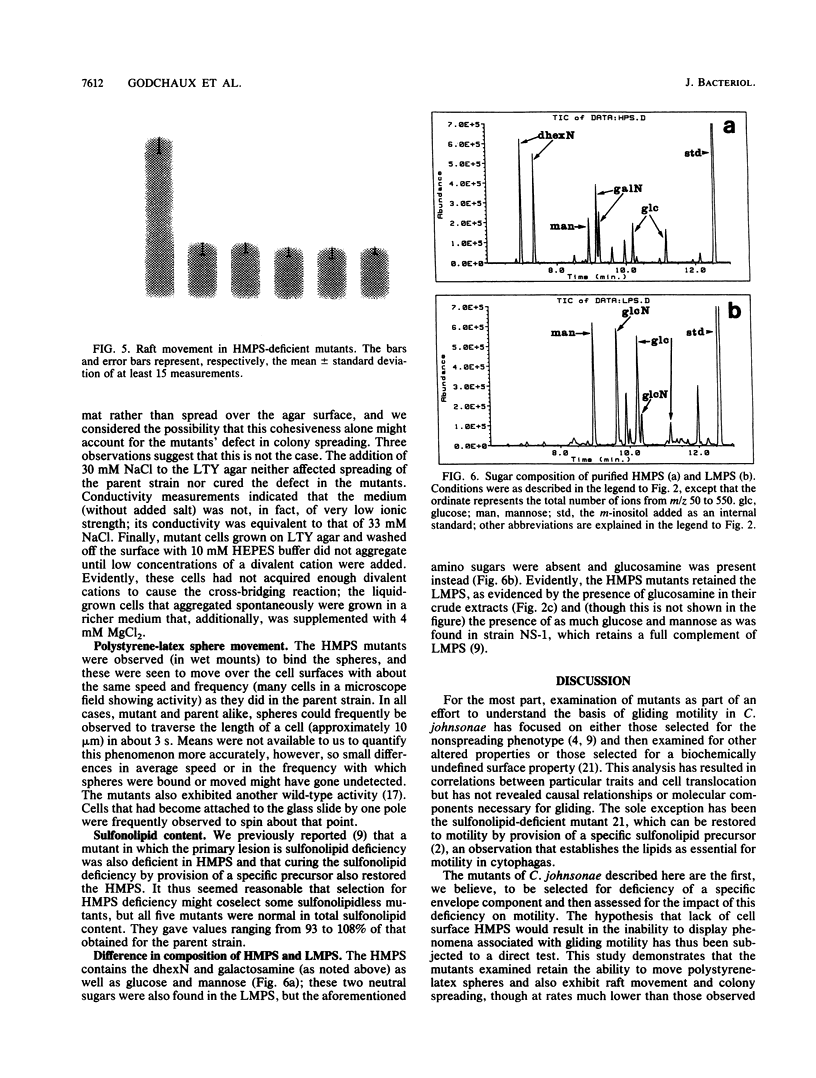


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burchard R. P. Gliding motility of prokaryotes: ultrastructure, physiology, and genetics. Annu Rev Microbiol. 1981;35:497–529. doi: 10.1146/annurev.mi.35.100181.002433. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Chang L. E., Pate J. L., Betzig R. J. Isolation and characterization of nonspreading mutants of the gliding bacterium Cytophaga johnsonae. J Bacteriol. 1984 Jul;159(1):26–35. doi: 10.1128/jb.159.1.26-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J. M., Zissler J. F. Defects in motility and development of Myxococcus xanthus lipopolysaccharide mutants. J Bacteriol. 1989 Apr;171(4):2042–2048. doi: 10.1128/jb.171.4.2042-2048.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Gorski L., Leadbetter E. R. Outer membrane polysaccharide deficiency in two nongliding mutants of Cytophaga johnsonae. J Bacteriol. 1990 Mar;172(3):1250–1255. doi: 10.1128/jb.172.3.1250-1255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Leadbetter E. R. Unusual sulfonolipids are characteristic of the Cytophaga-Flexibacter group. J Bacteriol. 1983 Mar;153(3):1238–1246. doi: 10.1128/jb.153.3.1238-1246.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus I. R., Berg H. C. Gliding motility of Cytophaga sp. strain U67. J Bacteriol. 1982 Jul;151(1):384–398. doi: 10.1128/jb.151.1.384-398.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau D. F., Melly M. A., Hash J. H. Surface polysaccharide from Staphylococcus aureus M that contains taurine, D-aminogalacturonic acid, and D-fucosamine. J Bacteriol. 1974 Sep;119(3):913–922. doi: 10.1128/jb.119.3.913-922.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Leadbetter E. R. Deoxyribonucleic acid base composition of myxobacteria. J Bacteriol. 1965 Dec;90(6):1795–1796. doi: 10.1128/jb.90.6.1795-1796.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M., Bryan L. E., Hancock R. E., McGroarty E. J. Heterogeneity of lipopolysaccharides from Pseudomonas aeruginosa: analysis of lipopolysaccharide chain length. J Bacteriol. 1988 Feb;170(2):512–521. doi: 10.1128/jb.170.2.512-521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]