Abstract
We have developed an immunotherapy in which tumor cells transfected with syngeneic major histocompatibility complex (MHC) class II genes are cell-based vaccines for the treatment of established tumor and metastatic disease. If this strategy is to be used clinically, convenient methods for generating class II+ tumor cells are necessary. Interferon-γ treatment or transduction of the class II transactivator (CIITA) gene induces class II expression but also up-regulates the class II-associated accessory molecules, invariant chain (Ii) and DM. To determine if interferon-γ treatment and CIITA transduction are potential immunotherapies, we assessed the tumorigenicity of sarcoma cells expressing combinations of class II, Ii, and DM. Since we hypothesized that class II-transfected tumor cells not coexpressing Ii and DM present endogenously encoded tumor peptides, we have assessed the transfectants for antigen presentation activity to MHC class II-restricted antigen-specific CD4+ T cells. Tumor challenge studies demonstrate that tumor cells expressing class II without coexpression of Ii or Ii plus DM are highly immunogenic and preferentially present endogenous antigens, while tumors coexpressing class II with Ii or Ii plus DM are not effective immunogens. Because tumor rejection correlates with expression of class II without coexpression of Ii and DM, the most efficacious vaccines will express MHC class II without coexpression of Ii and DM and will preferentially present endogenous antigen.
Numerous strategies for enhancing the immune response to autologous tumors have recently been developed. Many of the approaches directly target the activation of tumor-specific CD8+ effector T lymphocytes, since these cells are efficient mediators of tumor-specific immunity (1). In contrast, we (2, 3) and others (4–6) have reasoned that improved generation of tumor-specific CD4+ T helper cells enables tumor-specific CD8+ T cells to function more efficiently and, therefore, have focused on activating CD4+ T cells. We have used gene transfection to express syngeneic major histocompatibility complex (MHC) class II genes in tumor cells so that the tumor cells can directly present tumor peptides to CD4+ T helper lymphocytes and bypass the need for host antigen-presenting cells (APC) and soluble tumor antigen. Immunization of autologous mice with class II-transfected tumor cells protects against subsequent challenges of wild-type (class II−) primary tumor in a sarcoma model (2), decreases metastatic disease in two melanoma models (7), and mediates regression of a wild-type, long-term established, solid tumor in a sarcoma model (8). T cell depletion experiments established that class II-transfected tumor cells stimulate tumor-specific CD4+ T cells because they are more immunogenic than wild-type class II− tumor cells (2, 8). Immunization with class II-transfected tumor cells, therefore, induces a potent tumor-specific immunity that could be exploited for immunotherapy.
To develop the CD4+ T cell activation strategy and methods for predicting its potential clinical application, we are exploring additional approaches to enhance MHC class II expression. The two most practical of these approaches, interferon γ (IFN-γ) treatment (9) and class II transactivator (CIITA) gene transduction (10), not only increase autologous MHC class II expression but also up-regulate class II-associated accessory molecules, such as invariant (Ii) chain and DM (11–13). If IFN-γ treatment and/or CIITA gene expression are to be considered as immunotherapeutic strategies for treating tumor-bearing patients, the effects of class II-associated accessory molecule expression on class II-transfected tumor cell immunogenicity should be determined. We have, therefore, generated and determined the tumorigenicity of various sarcoma transfectants expressing different combinations of syngeneic MHC class II, Ii, and DM. Since we originally hypothesized that class II-transfected tumor cells that do not coexpress class II accessory molecules will present endogenously synthesized tumor peptides (2, 3), we have also assessed the ability of the various transfectants and transductants to present MHC class II-restricted antigen to antigen-specific CD4+ T cells. Our results demonstrate that tumor cells expressing syngeneic MHC class II without coexpression of Ii and DM are the only transfectants/transductants that induce tumor-specific immunity and efficiently present class II-restricted, endogenously synthesized antigens. Tumor cell coexpression of Ii and DM along with class II, therefore, abrogates the protective effects of class II expression, probably by preventing tumor cells from presenting endogenously synthesized tumor antigen(s). If cell-based vaccines using class II-transfected tumor cells are to be used as immunotherapeutic agents to generate tumor-specific CD4+ T cells, then the most effective vaccines will express MHC class II molecules and will not coexpress Ii and DM molecules.
EXPERIMENTAL PROCEDURES
Mice and Tumor Challenges.
All mice were purchased from The Jackson Laboratory and/or bred in the University of Maryland Baltimore County animal facility. For tumor challenges, mice were inoculated intraperitoneally (i.p.) with 106 tumor cells. This dose was chosen on the basis of previous in vivo tumor cell titrations (2, 14). After tumor challenge, mice were checked for tumor incidence 2–3 times per week, and sacrificed when moribund. Tumor-challenged mice were observed for up to 3 months and were considered tumor-free if they did not develop a palpable tumor during the observation period. Tumor incidence is the number of mice with tumor divided by the total number of mice injected.
Cell Lines.
SaI, SaI/Ak, and SaI/Ak/Ii transfectants were generated as described (2, 15). All tumor cells, transfectants, and transductants were maintained in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 5% Fetalclone I (HyClone), 1% penicillin, and 1% streptomycin. Additionally, SaI/Ak, SaI/Ak/Ii, and SaI/CIITA transfectants were maintained in 400 μg/ml G418, and lysozyme transfectants, in 400 μg/ml hygromycin. Cells transfected with both pSV2neo and pSV2hph plasmids were maintained in G418 and hygromycin. SaI/Ak/Ii cells were periodically enriched for class II expression by magnetic bead separation (Dynal).
Plasmids and Retrovirus Preparation.
Plasmid pKLK contains a viral promoter driving the hen eggwhite lysozyme (HEL) gene linked to the MHC class I transmembrane region of H-2Kb (16). This construct was used to generate SaI sarcoma cells expressing a membrane-bound HEL (mHEL). Plasmid pHYK contains a viral promoter driving the HEL gene fused to a KDEL endoplasmic reticulum (ER) retention signal (erHEL) (17). These plasmids were the generous gifts of C. Goodnow and H. Pelham, respectively. Plasmid pSV2hph contains the hygromycin phosphotransferase gene (14). The extrachromosomal expression vector BCMGhph (18) was kindly provided by D. Pardoll. Plasmid BCMGhph-erHEL was generated by subcloning a blunt-ended HindIII/BamHI fragment containing the HEL gene from the pHYK plasmid into the unique XhoI site of the BCMGhph vector.
For the CIITA construct, the EcoRI/EcoRI fragment of the pcDNA3.FLAG.CIITA construct (58) was cloned into the EcoRI site of the LXSNb retroviral plasmid, and production of retrovirus was as described (19).
Transfectants and Transductants.
Transfections were performed as previously described (15) using 25–50 μl of Lipofectin (GIBCO) and 0.2–1 μg of pSV2hph plus 10–30 μg of pKLK per 106 cells in 3 ml for mHEL or 10 μg of BCMGhph-erHEL per 106 cells in 3 ml for erHEL transfectants. Transfectants were selected using 400 μg/ml hygromycin (Calbiochem). mHEL transfectants were cloned by limiting dilution.
For retroviral infection, 105 SaI cells in mid-logarithmic growth phase were incubated for 2 hr with 250 μl of viral supernatant containing 8 mg/ml Polybrene. After incubation, the viral supernatant was replaced with fresh growth medium. Two days later the cells were passaged 1:20 and placed in selection (400 μg/ml G418). The polyclonal population was then analyzed for class II expression and cloned by limiting dilution.
Antigen-Presentation Assays.
Antigen-presentation assays were performed in 96-well flat-bottomed plates in a total volume of 300 μl per well of IMDM supplemented with 10% heat-inactivated Fetalclone I, 1% penicillin, and 1% streptomycin. HEL-(46–61)-specific, I-Ak-restricted 3A9 hybridoma cells (20) were irradiated with 2,200 rads (1 rad = 0.01 Gy) and mixed (5 × 104 per well) with SaI-derived APC (23,000 rads) or irradiated (2,400 rads) TA3 control B lymphoma cells (21). In initial experiments, ratios of 1:1, 2:1, and 5:1 3A9:APC were used. A ratio of 1:1 3A9:APC was used in subsequent experiments because it consistently gave the highest stimulation values. Cells were incubated at 37°C for 24 hr, and 50-μl samples of the supernatants were either frozen or assayed immediately for interleukin 2 (IL-2). IL-2 was measured by ELISA using an Endogen (Cambridge, MA) IL-2 kit and following the instructions of the supplier. For exogenous antigen presentation, intact or trypsin-digested HEL (22) was added to each well to a final concentration of 1 mg/ml. All wells were prepared in triplicate, and the average and standard deviation for each experimental condition were determined. Standard deviations usually did not exceed 5–10%.
Antibodies.
Hybridoma cell lines secreting the lysozyme-specific mAbs HyHEL 7 and HyHEL 10 (23) were kindly supplied by S. Smith-Gill and were affinity purified on protein G as described (2). The MHC class I (11–4.1: H-2Kk-specific) and invariant chain (In-1) mAbs have been described (2, 15). 10–2-16 is an MHC class II I-Ak-specific mAb (24). The polyclonal rabbit antiserum K553 against mouse DM (H-2M) was kindly supplied by L. Karlsson (25).
Immunofluorescence.
Live cells were stained by indirect immunofluorescence for cell surface markers (MHC class II, class I, mHEL) as described (2), using the 10–2-16, 11–4-1, or a cocktail of HyHEL 7 plus HyHEL 10 mAbs. Cells stained for internal markers (Ii, erHEL, DM) were fixed in 1% or 2% paraformaldehyde, permeabilized with 0.2% saponin, and then stained as for live cells (2) except 0.2% saponin was included in the wash medium and in all diluted reagents. erHEL transfectants were stained with a cocktail of HyHEL 7 plus 10 mAbs followed by a goat anti-mouse IgG-fluorescein isothiocyanate (FITC) conjugate (Cappel). Ii transfectants were stained with the In-1 mAb followed by an F(ab′)2 goat anti-rat IgG-FITC conjugate (Cappel). CIITA transductants were stained with the polyclonal rabbit anti-DM (no. K553) antiserum followed by an F(ab′)2 goat anti-rabbit IgG-FITC (Cappel). Cells were analyzed on a Coulter XL flow cytometer.
RESULTS
Sarcoma Tumor Cells Transfected with Syngeneic MHC Class II Genes Are Rejected by Autologous or Semisyngeneic Mice, While Sarcoma Cells Expressing Class II plus Ii Chain, With or Without DM, Are Highly Malignant.
Tumor expression of autologous MHC class II molecules can be increased by several methods, including transfection or transduction of class II genes (2), incubation of tumor cells with IFN-γ (9), or expression of the CIITA gene (10, 12, 26–29). Although the latter two approaches induce MHC class II expression, they also induce expression of the class II-associated accessory molecules Ii and DM. To determine if coexpression of Ii and DM with class II affects the therapeutic value of class II-transfected tumor cells, we have generated transfectants/transductants expressing class II genes and various combinations of Ii and DM and have tested their immunogenicity.
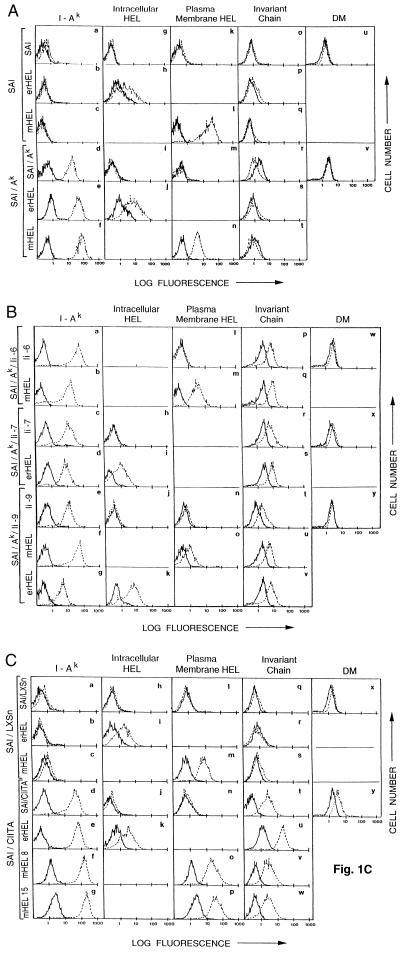
Fig. 1 shows flow cytometry profiles of wild-type SaI sarcoma cells, SaI cells expressing syngeneic MHC class II Aαk and Aβk genes (SaI/Ak tumor cells), SaI/Ak cells expressing Ii (SaI/Ak/Ii tumor cells), and SaI cells transduced with the CIITA gene (SaI/CIITA tumor cells). Tumor cells were stained by indirect immunofluorescence for syngeneic MHC class II molecules (I-Ak), Ii, or DM. Cell-surface MHC class II expression was assessed on live cells, while internal Ii and DM expression was assessed in fixed cells. As shown in Fig. 1A, wild-type SaI cells do not express I-Ak, Ii, or DM (panels a, o, or u, respectively), while SaI/Ak cells express I-Ak but not Ii or DM (panels d, r, and v, respectively). As shown in Fig. 1B, three clones of SaI/Ak/Ii cells (clones 6, 7, and 9) express I-Ak (panels a, c, and e) and intracellular Ii (panels p, r, and t) but not DM (panels w, x, and y). SaI/CIITA cells express I-Ak, Ii, and DM (Fig. 1C, panels d, t, and y), while vector-alone-transduced SaI cells (SaI/LXSN) do not express class II, Ii, or DM (Fig. 1C panels a, q, and x.) MHC class I levels on these cells are comparable to class I levels on SaI sarcoma cells (data not shown, but see refs. 2 and 15).
Figure 1.
Expression of MHC class II, Ii, and HEL in SaI and SaI-transfected tumor cells. (A) SaI and SaI/Ak sarcoma cells and their transfectants were stained for MHC class II (a–f), ER-retained HEL (g–j), plasma membrane HEL (k–n), Ii (o–t), or DM (u and v). (B) Three SaI/Ak/Ii transfected sarcoma lines (Ii-6, Ii-7, Ii-9) were stained for MHC class II (a–g), ER-retained HEL (h–k), plasma membrane HEL (l–o), Ii (p–v), or DM (w–y). (C) SaI/LXSN or SaI/CIITA sarcoma cells and their transfectants were stained for MHC class II (a–g), ER-retained HEL (h–k), plasma membrane HEL (l–p), Ii (q–w), or DM (x and y). In all panels, broken lines represent specific antibody staining; solid lines represent secondary fluorescent conjugate staining without primary antibody. MHC class II (10–2.16 mAb) and plasma membrane HEL (HyHEL 7, HyHEL 10 mAbs) staining was performed on live cells; internal HEL (ER-retained) and Ii chain staining was performed on fixed cells.
In previous studies, immunotherapeutic potential of SaI transfectants correlated with their tumorigenicity (2, 8, 15, 30). To test the immunogenicity of the transfectants and transductants, mice were challenged i.p. with tumor cells and monitored for ascites tumor growth and survival. Both autologous (A/J) and semisyngeneic [(C57BL/6 × A/J)F1] mice were used because previous experiments have established that SaI tumor growth is the same in both strains (ref. 31 and T.D.A., unpublished results). As shown in Table 1, wild-type class II− SaI, SaI/Ak/Ii, and SaI/CIITA tumor cells are highly tumorigenic. SaI/Ak/Ii lines have been tested for tumorigenicity in A/J mice, and they were found to form tumors in 50–100% of tested animals (15). SaI/Ak tumor cells, however, are uniformly rejected. These results agree with earlier findings that class II-transfected tumor cells are highly immunogenic in autologous hosts, while tumor cells coexpressing Ii are lethal. Optimal immunogenicity and tumor rejection, therefore, occur when sarcoma cells express syngeneic MHC class II molecules and do not coexpress class II-associated accessory molecules.
Table 1.
Sarcoma cells expressing syngeneic MHC class II molecules are rejected, while sarcoma cells coexpressing class II plus accessory molecules are lethal in autologous and semi-syngeneic mice
| Tumor cells | Dose | Tumor incidence
|
|
|---|---|---|---|
| A/J | (C57BL/6 × A/J)F1 | ||
| SaI | 106 | 2/2 | 10/10 |
| SaI/Ak | 106 | 0/5 | 0/5 |
| SaI/Ak/Ii-6 | 106 | NT | 4/5 |
| SaI/Ak/Ii-7 | 106 | NT | 8/9 |
| SaI/Ak/Ii-9 | 106 | NT | 8/10 |
| SaI/LXSN | 106 | 5/5 | 5/5 |
| 5 × 105 | 3/5 | ||
| 105 | 1/5 | ||
| SaI/CIITA | 106 | 5/5 | 5/5 |
| 5 × 105 | 4/5 | ||
| 105 | 5/5 | ||
The indicated number of sarcoma cells were inoculated i.p. into autologous A/J or semisyngeneic (C57BL/6 × A/J)F1 mice, and the mice were followed for tumor incidence. NT, not tested. Institutional Animal Care and Use Committee guidelines mandate that moribund animals be sacrificed to minimize pain and discomfort. On the basis of previous studies, mean survival times of these mice would have been 14–19 days.
SaI Tumor Cells Transfected with the HEL Gene Express Lysozyme in the Appropriate Targeted Cellular Compartment.
We originally hypothesized that class II-transfected tumor cells that do not coexpress Ii and/or DM would present endogenously synthesized tumor peptides and be effective APC that activate CD4+ Th (helper) lymphocytes (2, 3). Such tumor cells should be significantly more immunogenic than wild-type class II− tumor cells. To determine if this hypothesis explains the differential tumorigenicity of the SaI, SaI/Ak, SaI/Ak/Ii, and SaI/CIITA tumors, we have assessed their relative ability to present MHC class II-restricted antigen to CD4+ T cells. Since our hypothesis is that class II-transfected tumor cells would be more efficient presenters of any class II-restricted endogenous antigen, SaI sarcoma cells were transfected with the HEL gene, and presentation of lysozyme as an endogenous antigen has been assessed. HEL serves as a convenient model antigen in these studies for two reasons: I-Ak-restricted hybridomas (20) and cloned lysozyme genes (17, 16) are available for measuring HEL presentation. Furthermore, sarcoma transfectants expressing HEL in the ER (erHEL) or plasma membrane (mHEL) have been generated, thereby mimicking some of the intracellular locations in which tumor antigens are localized.
As shown in Fig. 1, SaI, SaI/Ak, SaI/Ak/Ii, and SaI/CIITA tumor cells transfected with the erHEL plasmid (SaI/erHEL, SaI/Ak/erHEL, SaI/Ak/Ii/erHEL, and SaI/CIITA/erHEL cells, respectively), or the mHEL plasmid (SaI/mHEL, SaI/Ak/mHEL, SaI/Ak/Ii/mHEL, and SaI/CIITA/mHEL cells, respectively) express lysozyme in the ER or at the plasma membrane (Fig. 1A, panels g–n; Fig. 1B, panels h–o; and Fig. 1C panels h–p). MHC class II (I-Ak), Ii, and DM expression in the transfectants is comparable to expression in the non-lysozyme-transfected tumors (Fig. 1). Cell lines transfected with vector alone show class II, Ii, and HEL levels comparable to untransfected parental cells (Fig. 1C and data not shown). MHC class I antigen expression in lysozyme transfectants is comparable to expression in untransfected cells (data not shown). Transfection of these tumor cells with the HEL gene, therefore, yields tumor cells that express lysozyme in the appropriate cellular compartment, and maintain MHC class II, class I, Ii, and/or DM expression comparable to their non-lysozyme-transfected relatives.
Class II-Transfected Tumor Cells Present ER-Retained Endogenously Synthesized HEL Provided They Do Not Coexpress Ii.
To determine if class II-transfected sarcoma cells present endogenously synthesized antigens, SaI/Ak/erHEL tumor cells were co-cultured with the I-Ak-restricted lysozyme-specific 3A9 hybridoma, and IL-2 secretion was measured. As shown in Table 2, SaI/Ak/erHEL cells stimulate IL-2 production, while control SaI/erHEL cells do not. SaI/Ak cells, therefore, express a functional MHC class II molecule, and present ER-retained endogenously synthesized antigen. To determine if Ii and DM coexpression affect presentation of ER-retained lysozyme, two SaI/Ak/Ii/erHEL clones and SaI/CIITA/erHEL cells were also tested as APC. As shown in Table 2, neither SaI/Ak/Ii/erHEL nor SaI/CIITA/erHEL cells cause IL-2 release. Class II+ tumor cells, therefore, present ER-retained antigen; however, coexpression of Ii or Ii plus DM prevents presentation.
Table 2.
SaI/Ak, but not SaI/Ak/Ii or SaI/CIITA, tumor cells present peptides derived from ER-retained lysozyme
| APC (erHEL-transfected) | IL-2, pg/ml
|
|||||
|---|---|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 5 | Exp. 6 | |
| SaI or SaI/LXSN | 0 | 0 | 0 | |||
| SaI/Ak | 79.2 | 47.5 | 85.8 | 594 | 198 | 99 |
| SaI/Ak/Ii-7 | 0 | 0 | 9.9 | |||
| SaI/Ak/Ii-9 | 0 | 0 | 0 | |||
| SaI/CIITA | 0 | 0 | 0 | |||
APC are transfectants expressing approximately equivalent levels of erHEL (see flow cytometry profiles in Fig. 1). APC were cocultured with 3A9 hybridoma cells, and IL-2 release was measured by ELISA. Six independent experiments are shown.
Class II-Transfected and CIITA-Transduced, but Not Ii-Transfected, Tumor Cells Present Membrane-Associated HEL.
To determine if class II-transfected sarcoma cells present endogenously synthesized antigen targeted to the plasma membrane, SaI/mHEL, SaI/Ak/mHEL, SaI/Ak/Ii/mHEL, and SaI/CIITA tumor cells were tested as APC to 3A9. As shown in Table 3, SaI/Ak/mHEL cells induce IL-2 synthesis, while control SaI/mHEL cells do not. Two clones of SaI/Ak/Ii/mHEL cells also do not stimulate IL-2 release, while SaI/CIITA/mHEL cells do. Class II-transfected sarcoma cells, therefore, present endogenously synthesized antigen targeted to the plasma membrane if class II is expressed alone, or coexpressed with Ii and DM; however, tumor cells expressing class II and Ii without DM do not present membrane-targeted antigen.
Table 3.
SaI/Ak and SaI/CIITA, but not SaI/Ak/Ii, cells effectively present membrane-associated HEL
| APC (mHEL-transfected) | IL-2, pg/ml
|
|||||
|---|---|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 5 | Exp. 6 | |
| SaI or SaI/LXSN | 0 | 0 | 0 | |||
| SaI/Ak | 151.8 | 330 | 316.8 | 405 | 514.8 | 138.6 |
| SaI/Ak/Ii-6 | 6.6 | 0 | 0 | |||
| SaI/Ak/Ii-9 | 0 | 0 | 0 | |||
| SaI/CIITA 8.27 | 990 | >1,749 | ||||
| SaI/CIITA 15.11 | 1,056 | 798.6 | ||||
All APC are transfectants expressing approximately equal quantities of mHEL (see Fig. 1 for flow cytometry quantitation). APC were incubated with HEL-specific 3A9 T cell hybridoma cells, and IL-2 release was measured by ELISA. Six independent experiments are shown.
Sarcoma Tumor Cells Do Not Present Exogenous Intact Lysozyme Unless They Are Transduced with the CIITA Gene.
We originally hypothesized that class II-transfected sarcoma cells are more immunogenic because they preferentially present endogenously synthesized tumor antigens. An alternative explanation for their increased immunogenicity is that they are efficient processors and presenters of exogenously synthesized antigen. To distinguish these two possibilities, SaI or SaI/Ak sarcoma cells were incubated with 3A9 hybridoma cells in the presence of exogenous intact HEL. TA3 cells, which are professional APC, were included as a reference standard. As shown in Table 4, neither SaI nor SaI/Ak cells stimulate IL-2 release, while TA3 cells consistently stimulate high levels of IL-2 release. Class II-transfected sarcoma cells are, therefore, unable to process and/or present exogenous intact antigen.
Table 4.
Only CIITA-transduced sarcoma cells efficiently present exogenous intact HEL
| APC | IL-2, pg/ml
|
||
|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 3 | |
| SaI | 0 | 0 | 0 |
| SaI/Ak | 0 | 0 | 52.8 |
| SaI/Ak/Ii-6 | 17.2 | ||
| SaI/Ak/Ii-7 | 14.5 | 0 | 0 |
| SaI/Ak/Il-9 | 0 | 0 | 0 |
| SaI/LXSN | 15 | ||
| SaI/CIITA | 376 | 171.6 | |
| TA3 | 3,696 | >1,386 | >3,234 |
APC were incubated with intact soluble HEL and 3A9 hybridoma cells, and IL-2 production was measured by ELISA. Three independent experiments are shown.
Since numerous studies have demonstrated that Ii and DM expression can have profound effects on presentation of exogenous antigen by class II+ APC (32, 33), we assessed presentation of exogenous antigen by sarcoma cells coexpressing class II, Ii, and Ii plus DM. SaI/Ak/Ii and SaI/CIITA cells were incubated with 3A9 hybridoma cells in the presence of exogenous intact HEL, and IL-2 production was measured. As shown in Table 4, three independent SaI/Ak/Ii clones and control SaI/LXSN sarcoma cells are unable to present exogenous HEL, while SaI/CIITA sarcoma cells present exogenous antigen, although the presentation is less efficient than by the professional APC, TA3. The ability to present exogenous intact antigen, is, therefore, limited to sarcoma cells that express class II, Ii, and DM.
Class II-Transfected Tumor Cells Present Low Levels of Exogenously Synthesized HEL Peptide.
As shown in Table 4, class II-transfected tumor cells do not present exogenously synthesized antigen unless they coexpress Ii and DM. Lack of presentation may be due to the cells’ inability to internalize and process antigen or to their inability to bind and present peptide. If class II-transfected tumor cells are unable to internalize and process exogenous antigen but are able to bind and present peptide, then transfectants given peptide antigen should activate antigen-specific hybridomas. To distinguish these possibilities, class II-transfected tumor cells were pulsed with trypsin-digested HEL in the presence of 3A9 hybridoma cells, and IL-2 production was measured. As shown in Table 5, SaI/Ak cells present lysozyme peptide better than they present intact lysozyme (compare with Table 4), but not as efficiently as the professional APC line, TA3. Since studies by other investigators have demonstrated that coexpression of Ii and DM significantly affects presentation of exogenous antigen (25, 34), we have also assessed SaI/Ak/Ii and SaI/CIITA as APC for exogenous lysozyme peptide. As shown in Table 5, one of the SaI/Ak/Ii lines does not present exogenous peptide, while the second SaI/Ak/Ii line and SaI/CIITA cells minimally present exogenous peptides. Class II-transfected sarcoma cells, therefore, are inefficient presenters of exogenous peptide relative to professional APC, and coexpression of Ii or Ii plus DM reduces presentation efficiency further.
Table 5.
Exogenous HEL peptides are presented most efficiently by SaI cells expressing MHC class II without coexpression of Ii and DM
| APC | IL-2, pg/ml
|
||
|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 3 | |
| SaI or SaI/LXSN | 0 | 0 | 0 |
| SaI/Ak | 39.6 | 211.2 | 151.8 |
| SaI/Ak/Ii-7 | 19.8 | 112.2 | 39.6 |
| SaI/Ak/Ii-9 | 0 | 0 | 33 |
| SaI/CIITA | 59.4 | 6.6 | 19.8 |
| TA3 | >1,333 | >1,472 | >2,746 |
APC, HEL, and 3A9 T cell hybridomas were incubated with trypsin-digested HEL. IL-2 release was measured by ELISA. Three independent experiments are shown.
DISCUSSION
Immunization of tumor-bearing mice with syngeneic MHC class II-transfected autologous tumor cells yields significant regression of established primary sarcoma tumors (8). If this approach is to be used clinically, it will be necessary to develop convenient methods for generating class II+ tumor cells. The two most practical methods, treatment with IFN-γ (9) and transfection/transduction of the CIITA gene (10), also up-regulate the class II-associated accessory molecules, Ii and DM (11–13), but our results demonstrate that tumor cells coexpressing class II with Ii, or Ii plus DM are not effective immunogens against tumor. IFN-γ or CIITA gene expression is, therefore, unlikely to be useful clinically. Furthermore, if cell-based vaccines aimed at stimulating tumor-specific CD4+ Th cells are to be adapted clinically, they should contain MHC class II molecules, and not coexpress Ii and DM.
Some human tumors either constitutively express MHC class II or are inducible for class II expression by IFN-γ (e.g., melanoma and mammary carcinoma). However, tumor cell class II expression does not always correlate with disease prognosis (35, 36). Since constitutively class II+ tumor cells and IFN-γ-treated tumor cells coexpress Ii and DM, these cells are probably not any more efficient at inducing a tumor-specific CD4+ T cell response than class II− tumor cells. Hence, in situ class II expression by tumor cells is unlikely to be a prognostic indicator for tumor regression.
Although the immunotherapeutic effect of class II-transfected sarcoma cells has been demonstrated (8, 14, 37), the mechanism of action has not been characterized. We originally hypothesized that class II+ Ii− tumor cell transfectants present endogenously encoded tumor antigen(s) in the context of self MHC class II to CD4+ T cells (3, 15, 37). This hypothesis is consistent with numerous published studies on the mechanism of class II-mediated antigen presentation, including (i) experiments showing that class II molecules present endogenously synthesized peptides (38–42), although they usually present exogenously synthesized antigens (43); (ii) demonstration that the MHC class II heterodimer binds endogenously synthesized antigen in the ER, provided Ii chain is not present (44); and (iii) sequence analysis studies demonstrating that endogenous peptides are bound to cell surface MHC class II dimers (45). Our studies are consistent with this hypothesis, since they demonstrate that class II-transfected sarcoma cells present class II-restricted endogenously encoded antigen.
An alternative mechanism for the increased immunogenicity of class II-transfected tumor cells is that they endocytose exogenous antigen and re-present it. Three observations argue against this mechanism: (i) Class II-transfected tumor cells do not present exogenous intact antigen (Table 4). (ii) Class II-transfected tumor cells are inefficient presenters of exogenous peptide (Table 5). (iii) SaI/CIITA tumor cells, which present exogenous intact antigen, are tumorigenic (Table 1). Therefore, presentation of exogenous antigen does not correlate with tumor cell immunogenicity, and re-presentation of exogenous antigen is unlikely to contribute to tumor rejection. Taken together, these data suggest that the most likely mechanism for enhanced immunogenicity of class II-transfected sarcoma cells is their ability to activate CD4+ T cells by means of cell surface expression of MHC class II/tumor peptide complexes.
Several studies suggest that coexpression of Ii is required for expression of functional MHC class II molecules (46–48). Other studies, however, including those reported here (Tables 2, 3, and 5), demonstrate that class II molecules are functional in the absence of Ii (39, 40, 44). Other studies suggest that I-Ak and I-Ek are less dependent on Ii coexpression for functional integrity (49). In addition to the tumors reported here, we have also transfected H-2b and H-2d tumors (melanoma B16 and mammary carcinoma 4T1, respectively) with syngeneic MHC class II genes and shown that these tumors stimulate tumor immunity (ref. 7; B. A. Pulaski and S.O.-R., unpublished results). Other investigators have transfected I-Ak or I-Ek class II genes into other tumors and shown functional expression of class II molecules in the absence of Ii (4, 50). Therefore, for at least three MHC haplotypes and five different tumors, transfection of MHC class II genes results in expression of functional class II molecules.
The immunotherapeutic strategy described here uses autologous tumor cells as the immunogen. Not all human malignancies will be amenable to this approach, since not all cancers can be cultured or transduced in vitro. However, phase I/II clinical trials using autologous tumor cells as immunotherapeutic agents are currently underway for a variety of human tumors [neuroblastoma, breast, melanoma, prostate, glioma, papilloma (http://cancernet.nci.nih.gov)]. Although the focus of our studies has been immunization with gene-modified tumor cells, we have also defined some of the molecules that facilitate T cell activation. By understanding which molecules effectively induce tumor immunity, one may be able to design more effective vaccines. Several scenarios may be possible if ex vivo autologous tumor cells are not available. Genes encoding known antigen-presentation molecules (i.e., MHC class II, B7, etc.) could be introduced in situ by viral transduction (51) or liposomes (52). Alternatively, autologous nontumor cells that are more easily manipulated in vitro (e.g., fibroblasts), transduced with the requisite antigen-presentation molecules and tumor antigen genes, could be used as vaccines. Since new tumor antigens are routinely being identified and characterized (53–55), and fibroblasts have been shown to be effective delivery vehicles (56, 57), this approach is theoretically feasible. Noncellular delivery methods, such as liposomes reconstituted ex vivo with the relevant tumor antigen peptides plus antigen-presentation molecules, could also be used. Regardless of the delivery approach, successful immunotherapy will require a full understanding of antigen-presentation molecules and tumor peptides that yield optimal T cell activation.
Acknowledgments
We thank Drs. C. Goodnow, D. Gerlier, S. Smith-Gill, L. Karlsson, and S. Adams for kindly providing gene constructs, mAbs, and cell lines, and Ms. Sandy Mason for her excellent care of our mice. This work was supported by National Institutes of Health Grants R01 CA52527 (S.O.-R.) and R01 CA48185 (J.P.-Y.T.) and National Research Service Award F32 CA67480 (B.K.M.).
ABBREVIATIONS
- MHC
major histocompatibility complex
- APC
antigen-presenting cells
- IFN-γ
interferon γ
- CIITA
class II transactivator
- HEL
hen eggwhite lysozyme
- mHEL
membrane-expressed HEL
- ER
endoplasmic reticulum
- erHEL
ER-expressed HEL
- Ii
invariant chain
- IL-2
interleukin 2
References
- 1.Pardoll D. Curr Opin Immunol. 1992;4:619–623. doi: 10.1016/0952-7915(92)90037-f. [DOI] [PubMed] [Google Scholar]
- 2.Ostrand-Rosenberg S, Thakur A, Clements V. J Immunol. 1990;144:4068–4071. [PubMed] [Google Scholar]
- 3.Ostrand-Rosenberg S. Curr Opin Immunol. 1994;6:722–727. doi: 10.1016/0952-7915(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 4.James R, Edwards S, Hui K, Bassett P, Grosveld F. Immunology. 1991;72:213–218. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen P, Ananthaswamy H. J Immunol. 1993;151:244–255. [PubMed] [Google Scholar]
- 6.Wu T-C, Guarnieri F, Staveley-O’Carroll K, Viscidi R, Levitsky H, Hedrick L, Cho K, August J T, Pardoll D. Proc Natl Acad Sci USA. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrand-Rosenberg S, Baskar S, Patterson N, Clements V. Tissue Antigens. 1996;47:414–421. doi: 10.1111/j.1399-0039.1996.tb02577.x. [DOI] [PubMed] [Google Scholar]
- 8.Baskar S, Glimcher L, Nabavi N, Jones R T, Ostrand-Rosenberg S. J Exp Med. 1995;181:619–629. doi: 10.1084/jem.181.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trinchieri G, Perussia B. Immunol Today. 1985;6:131–136. doi: 10.1016/0167-5699(85)90080-5. [DOI] [PubMed] [Google Scholar]
- 10.Steimle V, Otten L, Zufferey M, Mach B. Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- 11.Momburg F, Koch N, Moller P, Moldenhauer G, Butcher G, Hammerling G. J Immunol. 1986;136:940–948. [PubMed] [Google Scholar]
- 12.Chang C H, Flavell R A. J Exp Med. 1995;181:765–767. doi: 10.1084/jem.181.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern I, Steimle V, Siegrist C, Mach B. Int Immunol. 1995;7:1295–1299. doi: 10.1093/intimm/7.8.1295. [DOI] [PubMed] [Google Scholar]
- 14.Baskar S, Ostrand-Rosenberg S, Nabavi N, Nadler L M, Freeman G J, Glimcher L H. Proc Natl Acad Sci USA. 1993;90:5687–5690. doi: 10.1073/pnas.90.12.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clements V K, Armstrong T, Baskar S, Ostrand-Rosenberg S. J Immunol. 1992;149:2391–2396. [PubMed] [Google Scholar]
- 16.Hartley J, Crosbic J, Brink R, Kantor A, Basten A, Goodnow C. Nature (London) 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 17.Munro S, Pelham H. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 18.Karasuyama H, Melchers F. Eur J Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 19.Olsen J C, Sechelski J. Hum Gene Ther. 1995;6:1195–1202. doi: 10.1089/hum.1995.6.9-1195. [DOI] [PubMed] [Google Scholar]
- 20.Johnson N, Cavland A, Allen P, Glimcher L. J Immunol. 1989;142:3298–3304. [PubMed] [Google Scholar]
- 21.Glimcher L, Hamano T, Asofsky R, Sachs D, Pierres M, Samelson L, Sharrow S, Paul W. J Immunol. 1983;130:2287–2294. [PubMed] [Google Scholar]
- 22.Rosloniec E, Gay D, Freed J. J Immunol. 1989;142:4176–4183. [PubMed] [Google Scholar]
- 23.Smith-Gill S, Lavoie T, Mainhart C. J Immunol. 1984;133:384–393. [PubMed] [Google Scholar]
- 24.Oi V, Jones P, Goding J, Herzenberg L, Herzenberg L. Curr Top Microbiol Immunol. 1978;81:115–129. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson L, Peleraux A, Lindstedt R, Liljedahl M, Peterson P A. Science. 1994;266:1569–1573. doi: 10.1126/science.7985028. [DOI] [PubMed] [Google Scholar]
- 26.Steimle V, Siegrist C A, Mottet A, Lisowska-Grospierre B, Mach B. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 27.Chin K-C, Mao C, Skinner C, Riley J L, Wright K L, Moreno C S, Stark G R, Boss J M, Ting J P Y. Immunity. 1994;1:687–697. doi: 10.1016/1074-7613(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 28.Chang C H, Fontes J D, Peterlin M, Flavell R A. J Exp Med. 1994;180:1367–1374. doi: 10.1084/jem.180.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mach B, Steimle V, Martinez-Soria E, Reith W. Annu Rev Immunol. 1996;14:301–331. doi: 10.1146/annurev.immunol.14.1.301. [DOI] [PubMed] [Google Scholar]
- 30.Ostrand-Rosenberg S, Roby C A, Clements V K. J Immunol. 1991;147:2419–2422. [PubMed] [Google Scholar]
- 31.Berendt M, North R, Kirstein D. J Exp Med. 1978;148:1560–1569. doi: 10.1084/jem.148.6.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadimi F, Moreno J, Momburg F, Heuser A, Fuchs S, Adorini L, Hammerling G. Eur J Immunol. 1991;21:1255–1263. doi: 10.1002/eji.1830210524. [DOI] [PubMed] [Google Scholar]
- 33.Brooks A, Campbell P, Reynolds P, Gautam A, McCluskey J. J Immunol. 1994;153:5382–5392. [PubMed] [Google Scholar]
- 34.Germain R. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Nevot M, Garcia E, Romero C, Oliva M, Serrano S, Garrido F. Exp Clin Immunogenet. 1988;5:203–212. [PubMed] [Google Scholar]
- 36.Cabrera T, Ruiz-Cabello F, Garrido F. Scan J Immunol. 1995;41:398–406. doi: 10.1111/j.1365-3083.1995.tb03584.x. [DOI] [PubMed] [Google Scholar]
- 37.Ostrand-Rosenberg S, Cole G A, Nishimura M I, Clements V K. Cell Immunol. 1990;128:152–164. doi: 10.1016/0008-8749(90)90014-i. [DOI] [PubMed] [Google Scholar]
- 38.Nuchtern J, Biddison W, Klausner R. Nature (London) 1990;343:74–76. doi: 10.1038/343074a0. [DOI] [PubMed] [Google Scholar]
- 39.Miller J, Germain R. J Exp Med. 1986;164:1478–1489. doi: 10.1084/jem.164.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sekaly R P, Tonnelle C, Strubin M, Mach B, Long E O. J Exp Med. 1986;164:1490–1504. doi: 10.1084/jem.164.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaraquemada D, Marti M, Long E. J Exp Med. 1990;172:947–954. doi: 10.1084/jem.172.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobson S, Sekaly R, Jacobson C, McFarland H, Long E. J Virol. 1989;63:1756–1762. doi: 10.1128/jvi.63.4.1756-1762.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X, Pierce S. J Immunol. 1995;155:1652–1654. [PubMed] [Google Scholar]
- 44.Busch R, Cloutier I, Sekaly R, Hammerling G. EMBO J. 1996;15:418–428. [PMC free article] [PubMed] [Google Scholar]
- 45.Rudensky A, Preston-Hurlburt P, Hong S, Barlow A, Janeway C. Nature (London) 1991;353:622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 46.Vivelle S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Cell. 1993;72:635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- 47.Elliott E, Drake J, Amigorena S, Elsemore J, Webster P, Mellman I, Flavell R. J Exp Med. 1994;179:681–694. doi: 10.1084/jem.179.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bikoff E, Huang L-Y, Episkopou V, Meerwijk J, Germain R, Robertson E. J Exp Med. 1993;177:1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bikoff E, Germain R, Robertson E. Immunity. 1995;2:301–310. doi: 10.1016/1074-7613(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 50.Chen P, Ullrich S, Ananthaswamy H. J Leukocyte Biol. 1994;56:469–474. doi: 10.1002/jlb.56.4.469. [DOI] [PubMed] [Google Scholar]
- 51.Mulligan R. Science. 1993;260:926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- 52.Nabel G, Gordon D, Bishop D, Nickoloff B, Yang Z, Aruga A, Cameron M, Nabel E, Chang A. Proc Natl Acad Sci USA. 1996;93:15388–15393. doi: 10.1073/pnas.93.26.15388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robbins P, Kawakami Y. Curr Opin Immunol. 1996;8:628–636. doi: 10.1016/s0952-7915(96)80078-1. [DOI] [PubMed] [Google Scholar]
- 54.Pardoll D. Curr Opin Immunol. 1996;8:619–621. doi: 10.1016/s0952-7915(96)80076-8. [DOI] [PubMed] [Google Scholar]
- 55.Boon T, Cerrotini J, Van Pel A, van der Bruggen P. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 56.Tahara H, Zeh H, Storkus W, Pappo I, Watkins C, Gubler U, Wolf S, Robbins P, Lotze M. Canc Res. 1994;54:182–189. [PubMed] [Google Scholar]
- 57.Kundig T, Bachmann M, DiPaolo C, Simard J, Battegay M, Lother H, Gessner A, Kuhlcke K, Ohashi P, Hengartner H, Zinkernagel R. Science. 1995;268:1343–1347. doi: 10.1126/science.7761853. [DOI] [PubMed] [Google Scholar]
- 58.Chin K-C, Li G, Ting J P-Y. Proc Natl Acad Sci USA. 1997;94:2501–2506. doi: 10.1073/pnas.94.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]