Abstract
We describe a general approach for achieving efficient and cell type-specific expression of exogenous genes in photoreceptor cells of the mammalian retina. Recombinant adeno-associated virus (rAAV) vectors were used to transfer the bacterial lacZ gene or a synthetic green fluorescent protein gene (gfp) to mouse or rat retinas after injection into the subretinal space. Using a proximal murine rod opsin promoter (+86 to −385) to drive expression, reporter gene product was found exclusively in photoreceptors, not in any other retinal cell type or in the adjacent retinal pigment epithelium. GFP-expressing photoreceptors typically encompassed 10–20% of the total retinal area after a single 2-μl injection. Photoreceptors were transduced with nearly 100% efficiency in the region directly surrounding the injection site. We estimate approximately 2.5 million photoreceptors were transduced as a result of the single subretinal inoculation. This level of gene transfer and expression suggests the feasibility of genetic therapy for retinal disease. The gfp-containing rAAV stock was substantially free of both adenovirus and wild-type AAV, as judged by plaque assay and infectious center assay, respectively. Thus, highly purified, helper virus-free rAAV vectors can achieve high-frequency tissue-specific transduction of terminally differentiated, postmitotic photoreceptor cells.
Keywords: gene therapy, retina, retinitis pigmentosa
Key elements in most gene therapy protocols include the safe, efficient, and stable transfer of the therapeutic gene and associated regulatory elements to the appropriate, usually the affected, cell type. Many inherited retinal degenerations may be amenable to direct gene transfer therapies, as the photoreceptor (PR) cell is the predominant, and often only, cell type involved. The best studied of this class of genetic disease is retinitis pigmentosa (RP), a clinically and genetically heterogeneous group of diseases characterized by progressive PR degeneration (1–3). The molecular pathogenesis of RP is still unexplained. Ultrastructural observations suggest that the rod PRs are severely affected in the disease (4). RP families have been documented with dominant, recessive, X-linked, and digenic patterns of inheritance, and more than 15 separate loci have been implicated by linkage studies (5). Currently, the mutations identified to date all occur in genes exhibiting a PR-specific pattern of expression. The etiology of this blinding disease leads us to the primary aim of this study, to achieve exclusive or predominant gene expression in PRs of the mammalian retina. Reaching this goal will be a major step in the development of a genetic therapy for RP.
Previous studies of in vivo gene transfer and expression in mammalian retina have used exclusively the immediate early cytomegalovirus promoter (6–10) for regulating reporter or therapeutic gene expression. In these studies, subretinal injections of either recombinant adenovirus or recombinant adeno-associated virus (rAAV) resulted in efficient, high-level expression of the lacZ reporter gene predominantly in retinal pigment epithelium (RPE) cells, with only sporadic, very low-efficiency PR expression. Therefore, one purpose of this study was to determine whether regulatory elements from a well established PR-specific gene, the rod opsin gene, would enhance PR transduction efficiency. In previous studies, analysis of transgenic mice with the rhodopsin promoter linked to the lacZ gene demonstrated that as little as 472 bp of mouse or 222 bp of bovine 5′ upstream sequence was sufficient for retinal expression (11, 12). In vitro analysis of transcriptional elements regulating rod opsin expression have identified a conserved element (ret-1) that is bound by a retina-specific protein in all mammals tested (13, 14) within a few hundred base pairs of the transcriptional start site. Guided by these results, we tested the same 472-bp mouse rhodopsin promoter previously used (11) and compared both bacterial LacZ and synthetic green fluorescent protein (GFP) expression patterns in rodent retina.
The choice of gene delivery technique was based on several considerations. First, recombinant viral vectors were deemed preferable because of the very high gene transfer efficiencies often achieved. Second, of the viral vectors well developed for gene transfer, recombinant adenovirus or rAAV are most often considered for in vivo experiments (15). Finally, AAV was the preferred vector because of its well established ability to transfect quiescent cells (16) and broad host range (17). This choice is not without controversy, however, because of recent suggestions that adenovirus gene expression (18, 19) or a variety of cellular DNA repair-inducing agents (20, 21) may enhance or even be required for efficient AAV-mediated transduction, particularly in nondividing cells. Additionally, wild-type AAV (wtAAV) could contaminate viral stocks and alter transduction efficiency. Therefore, the second purpose of this study was to establish whether an rAAV vector demonstrably free of infectious adenovirus or wtAAV is capable of efficient in vivo transduction of a targeted postmitotic cell, the photoreceptor.
MATERIALS AND METHODS
rAAV Plasmid Construction.
The mOp-lacZ-rAAV plasmid DNA was made by first inserting the 4.3-kbp BglII/BamHI fragment containing the proximal murine rod opsin promoter (+86 to −385) and the entire lacZ gene of clone pRG3 (11) into the BglII sites of pTR, which contains the AAV TR sequences and a simian virus 40 polyadenylylation sequence (Fig. 1a). The mOp-gfp-rAAV plasmid DNA was made by first adding NotI linkers to the 472-bp BglII/XhoI proximal opsin promoter fragment of pRG3 and inserting it into the NotI sites of pTRUF2 (22) (Fig. 1b).
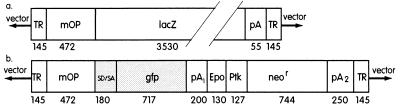
Figure 1.
Vector construction. Schematic diagram of the plasmid DNA constructs used to make rAAV viruses mOp-lacZ (a) and mOp-gfp (b). TR, 145-bp AAV terminal repeat sequence; mOp, 472-bp murine rod opsin regulatory sequence from +86 to −385; SD/SA, 180-bp simian virus 40 late viral protein gene 16S/19S splice donor and acceptor signal; lacZ; coding sequence for the bacterial lacZ gene; gfp, coding sequence for the synthetic green fluorescence gene; pA, pA1, and pA2, polyadenylylation signals; Epo, a tandem repeat of the polyoma virus enhancer region (bases 5,210–5,274); Ptk, thymidine kinase promoter of herpesvirus (bases 92–218); neor, coding sequence of the neomycin resistance gene, Tn5 (bases 1,555–2,347) (22).
rAAV Virus Production and Analysis.
To generate recombinant virus, human 293 cells were cotransfected with mOp-lacZ-rAAV or mOp-gfp-rAAV plasmid DNA and the helper pIM45 plasmid DNA carrying the wtAAV genome without terminal repeats (22). Cultures were then infected with helper adenovirus Ad-ts149 for the lacZ virus or with Ad5 for the gfp virus, at a multiplicity of infection of 10. rAAV and wtAAV titers were determined by infectious center assay (23), which is independent of the transgene or opsin promoter used. Titers of contaminating adenovirus were determined by plaque assay for mOp-gfp-rAAV and by serial dilution cytopathic effect for mOp-lacZ-rAAV. Adenovirus was not detectable in either of the rAAV preparations (Table 1).
Table 1.
Titers of rAAV viruses and potential contaminants
| Virus | rAAV titer, icu/ml | Adenovirus titer, pfu/ml | wtAAV titer, icu/ml |
|---|---|---|---|
| mOps-lacZ | 1 × 107 | Undetectable | <103 |
| mOp-gfp | 4.6 × 109 | <103 | <103 |
rAAV and wtAAV were titered by infectious center assay. For wtAAV, 1 μl of the undiluted rAAV stock was titered (pfu, plaque-forming unit). Adenovirus was titered by plaque assay using 1 μl of the undiluted rAAV stock. No infectious adenovirus was detected by serial passage cytopathic effect.
Subretinal Injection of rAAV.
Thirty adult C57BL/6J (The Jackson Laboratory) pigmented mice between 3 and 6 months of age and 27 adult albino Sprague–Dawley rats between 3 and 4 months of age were used. Animals were anesthetized by ketamine/xylazine injection, eyes were dilated (2.5% phenylephrine and 0.5% tropicamide), and a local anesthetic (proparacain HCl) was applied. Injections (1 μl in mice and 2 μl in rats) were made into the right eye with blunt 32-gauge needles through an opening in the pars-plana, delivering the rAAV suspension into the superior subretinal space. Control injections were made in the contralateral eye with PBS only. Injections were performed with an operating microscope, and the subretinal location of the injected volume was confirmed by ophthalmoscopy.
Tissue Analysis.
Animals were euthanized by intramuscular injection of ketamine, followed by pentobarbital overdose. The eyes were immediately enucleated and the site of virus injection marked. The cornea, lens, and vitreous of each eye were removed, and the posterior eyecup placed in primary fixative.
For β-galactosidase staining, eyecups were fixed in 0.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.5, for 15 min at room temperature. After a 10-min wash in PBS, the eyecups were incubated in an iron-based 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) staining solution (24) in a shaking water bath at 35°C for 12 hr. For agarose embedment, retinas were detached from the RPE, submerged without dehydration in molten 5% agarose, and cooled to 25°C. Retinas were sectioned in the transverse axis in isotonic PBS on a Vibratome at 50–100 μm. Bright-field and phase-contrast micrographs of whole mounts and β-galactosidase-stained sections were made with a Zeiss Axiophot.
GFP fluorescence was examined in retinal whole mounts and agarose-embedded sections. Tissue fixation was minimized to reduce retinal autofluorescence. Retinas were detached from eyecups, fixed for 15 min at room temperature in 4% formaldehyde, 0.1 M PO4 buffer, pH 7.5, and rinsed three times in PBS. Whole mounts were photographed with epifluorescence using Zeiss filter set 09 (excitation 450–490 nm, barrier 510 nm, emission 520 nm) and an AttoArc (Zeiss) variable output UV lamp to minimize GFP bleaching. Whole-mount retinas then were embedded in agarose as above for 100-μm transverse Vibratome sections, and fluorescence was documented as for the whole mount. Higher resolution images were collected with a Molecular Dynamics confocal microscope (Nikon 40× or 60× 1.4 n.a. oil objectives; argon laser excitation at 514 nm, emission at 520–560 nm). Optical sections were made in 0.32-μm steps. Full frame (768 × 512) eight-bit images were collected and processed with Adobe Photoshop. Area measurements were made with National Institutes of Health Image analysis software (25).
Expression of the lacZ reporter gene in murine retinal cells was analyzed by reverse transcriptase–PCR (RT-PCR). Pieces of retina (1 mm2), were detached from unfixed eyecups and dissected free of RPE, homogenized with a pestle fitted to a 1.5-ml tube and total RNA isolated using the trizol reagent (phenol/guanidine isothiocyanate, GIBCO/BRL) according to the manufacturer’s recommendations. The RNA was additionally purified over an RNA easy-spin column (Qiagen). The RT-PCR used a two-buffer thermostable Tth polymerase system (Promega) according to manufacturer’s instructions and lacZ sequence primers from nucleotides 105–124 (forward) and 303–286 (reverse). RNase and DNase digestions before the RT-PCR were performed as previously described (26).
RESULTS
Design of rAAV Vectors for Gene Transfer to Photoreceptors.
To express a foreign gene specifically in the mammalian PR by AAV-mediated delivery, we linked 472 bp of the proximal murine rod opsin promoter (+86 to −385) to a lacZ-simian virus 40 poly(A)+ reporter gene and then inserted this into pTR. The gene construct was packaged into AAV virus particles, concentrated, tested for contaminating adenovirus, and titered for rAAV by an infectious center assay (Table 1). The right eyes of 30 C57BL/6J mice were injected subretinally with 1 μl of mOp-lacZ virus (107 infectious units per ml). After 2 weeks, the right (test) and left (control) eyes of 12 animals were removed, fixed, and stained with X-Gal. Test retina in 6 of 12 injected eyes exhibited a focal blue region (Fig. 2a) consistent with a subretinal bleb of the injected virus creating a localized retinal detachment. All control eyes showed no X-Gal reaction. Reporter gene expression was examined in mice sacrificed at later periods and was detected at 10 weeks postinjection, suggesting persistent reporter transgene expression.
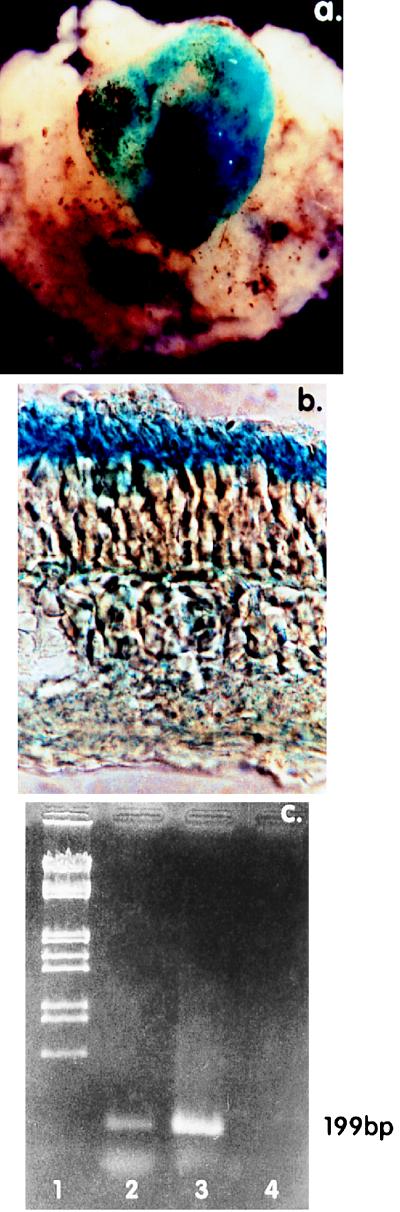
Figure 2.
lacZ expression in the mouse retina. (a) Mouse retina reacted with X-Gal 2 weeks after subretinal injection with 1 μl of the AAV vector. Whole mount of retina everted after detachment from RPE. (50× magnification.) (b) Agarose section of the mouse retina (a) (150× magnification.) (c) RT-PCR analysis of lacZ expression in PRs of the retina in a. Ethidium bromide-stained gel of products of a RT-PCR performed on total cellular RNA extracted from retinal homogenates, 2 weeks after rAAV administration. Lane 1: Size marker. Lane 2: RT-PCR product using lacZ specific primers 199 bp apart. The 199-bp product is indicated by the arrow. Similar RT-PCR from uninjected retinas were negative (not shown). Lane 3: As in lane 2 except RNase-free DNase treatment preceded the RT step. Lane 4: As in lane 2 except RNase treatment preceded the RT step.
LacZ and GFP Reporter Genes Are Expressed Exclusively in Photoreceptors.
We analyzed the distribution of lacZ gene product at higher resolution by preparing serial 50-μm transverse sections from the entire whole mounts. A section through the blue region observed in the whole mount is shown in Fig. 2b. The blue X-Gal reaction product is observed primarily in the PR inner segments (Fig. 2b). Most of the PRs were filled with X-Gal in this region. X-Gal staining was slightly above control levels in the PR synaptic termini in the outer plexiform layer. PR outer segments, RPE, and other retinal cells in this region did not reveal X-Gal staining above baseline levels observed in identically treated, uninjected, or PBS-injected control retinas from the contralateral eye. Examination of additional transverse sections confirmed that the region of positive staining radiated outward from the injection site in a progressively reducing fraction of PR inner segments until baseline levels were seen. The area of X-Gal-positive PRs was consistent with the blue area in the whole-mount view (Fig. 2a). Neural retina and RPE were separated and analyzed independently to control for the possibility that the β-galactosidase enzyme or its X-Gal reaction product was transferred from transduced RPE cells to PRs (8). Total mRNA was extracted from neural retina, and RPE from injected animals and tested for the presence of lacZ mRNA by RT-PCR (Fig. 2c). The 199-bp amplification product diagnostic for lacZ RNA (nucleotides 105–303) can be seen when total RNA from a portion of a mouse retina sacrificed at 2 weeks postinjection is amplified. The amplification template was a cellular RNA because of its resistance to DNase pretreatment and sensitivity to RNase pretreatment. The remaining RPE tissue was negative for this RT-PCR product (not shown). This demonstrates that the observed X-Gal product was derived from β-galactosidase expression within PR cells and not derived from RPE expression. Although the isolation protocol eliminates cellular DNA, it is likely that rAAV DNA is in both PR and RPE because we previously demonstrated the rAAV-mediated transduction of RPE (22).
A second reporter gene, a synthetic version of the Aequorea victoria green fluorescent gene (gfp) (22) was used to independently confirm the apparent cell-type specificity of transduction. We used the same murine rod opsin promoter and an analogous rAAV vector to construct the mOp-gfp virus (Fig. 1b). Two microliters of gfp-containing rAAV was injected into the subretinal space of eight Sprague–Dawley rats. Rats were used in place of mice because the larger eye allowed more reproducible subretinal inoculations. Retinal whole mounts prepared from all eight rat eyes that were injected contained a fluorescent region of superior retina surrounding the site of inoculation. GFP fluorescence typically extended over 10–20% of the retinal area in a radial pattern from the injection site (Fig. 3a). Immediately surrounding the point of injection (red arrow), the transduction frequency, as judged by the intensity of GFP fluorescence, was very high, with a continuous positive signal. In transverse sections extending from the central retina to the periphery (Fig. 3b), beyond a region of apparently saturated GFP fluorescence, the percentage of transduced cells decreased radially with distance from the injection site. GFP-positive cells were easily identifiable as PRs by their specialized shape and location in the retina. Hence, only PR cells appeared to have been transduced, i.e., infected by the rAAV and expressing the gfp passenger gene.
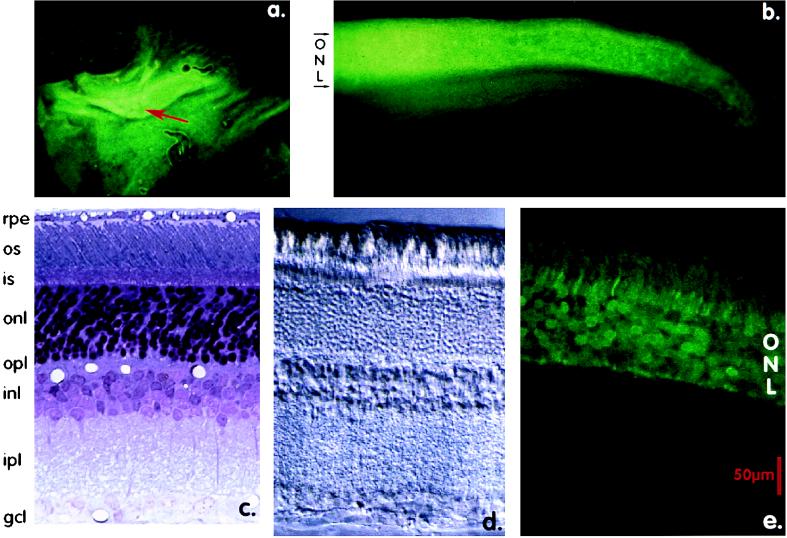
Figure 3.
GFP Expression in rat retina. (a) Whole mount of rat retina 6 weeks after subretinal injection of 2 μl of AAV-pmOp-gfp virus. Cells in an oval area of several millimeters surrounding the injection site in the superior retina are GFP-positive. The percentage of GFP-positive cells decreases radially surrounding the injection site. (6× magnification). (b) Transverse section of the rat retina shown in a. GFP fluorescence is seen throughout the outer nuclear layer extending toward the ora seratta. No GFP fluorescence is observed in cells of the inner layers of the neurosensory retina. (100× magnification). (c) Bright-field micrograph of a rat retina for reference. (2,500× magnification). OS, outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; GCL, ganglion cell layer. (d) Phase-contrast micrograph of 100-μm thick agarose section of rat retina. Retinal layers and locations of the cell nuclei can be observed for comparison to e. (2,500× magnification.) (e) Laser scanning confocal image of the rat retina shown in a, b, and d. Diffuse GFP fluorescence is observed filling the PR cell bodies extending from the synaptic terminal to the region of the external limiting membrane. The degree of GFP fluorescence decreases significantly above the connecting cilium to levels not significantly above autofluorescence in the outer segment. (Bar = 50 μm.)
Opsin Promoter Confers Photoreceptor-Cell Specificity.
The PR-specific pattern of GFP expression was confirmed by laser confocal microscopy (Fig. 3e). GFP was not observed between the inner limiting membrane (vitreal face of the inner retina) and the outer plexiform layer (junction of the inner retina with PR synaptic termini), as indicated in Fig. 3e. This region contains all the non-PR retinal neuroal (bipolar, horizontal, amacrine, and ganglion) and glial (Müller) cells. A micrograph of a conventionally fixed rat retina and a phase-contrast micrograph of a section through this fluorescent region of the retina are shown in Fig. 3 c and d for reference. In contrast, virtually 100% of the PR inner segments, cell bodies, and synaptic terminals (Fig. 3 b and e) exhibited strong GFP fluorescence. In regions more peripheral to the injection site (Fig. 3b), the fraction of positive PRs was substantially reduced, consistent with the radial decline in fluorescence seen in retinal whole mounts (Fig. 3a). We established that all PR cell bodies contained GFP signal by examining serial optical sections (0.32 μm). Through-focus series demonstrated that occasional, dark regions in the outer nuclear layer seen in Fig. 3e always contained a GFP-positive PR cell body in another plane of section. Therefore, all PRs, including both rods and cones, supported reporter gene expression. Outer segments demonstrated less fluorescence than other PR compartments, near the level of autofluorescence seen in control outer segments. No GFP signal was observed in the RPE, choroid, or sclera (not shown).
rAAV Transduces PRs with High Efficiency.
We estimated the area of GFP-positive PRs resulting from a typical injection from epifluorescence images of retinal whole mounts. GFP-positive areas were measured with National Institutes of Health Image software by segmenting the image into regions of GFP fluorescence and background on the basis of gray level. Area measurements were calibrated by imaging a 1,000-μm reference scale on the film together with the whole mount.
We measured the retinal area that contained 50% or more PR cells positive for GFP signal in whole mounts as shown in Fig. 3 b and e. On average, the GFP-positive area covered ≈35% of the total retinal area of the rat retina. We estimated the number of GFP-positive PRs resulting from a typical injection by examining serial optical sections taken through the retina. Serial confocal images as in Fig. 3e suggest 100% PR transduction in the region directly adjacent to the injection site, because we did not observe GFP-negative cell bodies within the outer nuclear layer in adjacent confocal optical sections. We estimate that the whole rat retina contains 15.7 million PR cells, from counts of total PR nuclei in representative images, as in Fig. 3e. From these observations, a conservative estimate is that 2–3 million PRs were transduced by the gfp-containing rAAV. Because there were 9.2 million infectious rAAV particles in the 2-μl injection volume, one PR cell was transduced for every 3–4 rAAV injected, a 25% transduction efficiency. This value needs to be viewed as an approximation, however, because infectious titers of rAAV were determined on human 293 cells, not rat PRs, and assayed by probe hybridization, not by GFP fluorescence.
Purified rAAV Transduces Photoreceptors in the Absence of Helper Viruses.
rAAV production currently requires the participation of both helper adenovirus and complementing but nonpackaging AAV rep and cap genes (15). This raises the possibility that adenovirus or wtAAV could contaminate the rAAV preparations and may participate in the efficient transduction observed for the gfp-containing virus. Indeed, recent experiments demonstrate both the recruitment of rAAV to intracellular centers of adenovirus replication (18) and the enhancement of rAAV transduction upon the expression of adenovirus gene products from the E4 domain (19). We attempted to estimate the infectious particles of adenovirus and wtAAV DNA in the mOps-gfp viral preparation by plaque assays (adenovirus) and infectious center assays (wtAAV) (Table 1). Both wtAAV and adenovirus infectious particles in the purified recombinant virus were below detection limits of 103 infectious centers or plaque-forming units per ml, respectively. Therefore, we expect less than one infectious particle of either contaminant per microliter of injected virus.
Slow Accumulation of GFP in Transduced Photoreceptors.
Comparison of retinal whole mounts and histologic sections at several times postinjection revealed a distinct, reproducible increase in GFP fluorescence as a function of the length of expression in vivo. PR cell bodies and inner segments were only weakly and sporadically GFP-positive at 1–3 weeks postinjection, whereas at 3–6 weeks and thereafter, GFP expression was robust and uniform in nearly all PRs near the injection site (Fig. 3e). This slow onset of measurable GFP signal contrasts to that seen in tissue culture cells (22) and has several implications, discussed below, for the mechanism of gene expression via AAV-mediated transduction.
DISCUSSION
Implications for Retinal Gene Therapy.
We tested the hypothesis that a 472-bp proximal murine rod opsin promoter would drive reporter gene expression specifically and efficiently in rodent PR cells. When delivered in an rAAV vector via subretinal injection, either bacterial β-galactosidase or synthetic GFP reporters were expressed in nearly 100% of PRs in a radial domain centered on the injection site and in essentially no other retinal cell type. This contrasts markedly with the very low efficiency (ca. <0.1%) of PR transduction observed by delivery of reporter genes driven by an immediate early cytomegalovirus promoter (6, 8, 9). The use of such proximal opsin promoters therefore could substantially enhance the efficiency of expression in cases where rod and/or cone-specific expression of a potentially therapeutic gene is desired.
The total number of transduced PR cells obtained with a given titer of recombinant virus vector is a key parameter in evaluating the potential of any regimen for correcting or ameliorating genetic defects. This is particularly important for the retina because of the need to transduce a substantial fraction of target cells using a minimal injection volume to minimize tissue damage. A single 2-μl subretinal injection of rAAV transduced approximately 2.5 million rat PRs over 15–20% of the total retinal area. Direct extrapolation to the human retina is not straightforward because of species differences in rod density patterns. Relative to the rat, the average density of human PRs per unit area is lower, and the rod density changes substantially as a function of distance from the cone-rich retinal center or fovea (27, 28). With these considerations in mind, an rAAV transduction estimate in humans can be made.
Assuming a 20-μl injection into the central human retina at the region of greatest rod density, approximately 8 million rods would be expected to be transduced in a focal region encompassing approximately 6% of the total retina (27, 29). Such an area of transduced PRs could improve or delay retinal degenerations in a variety of inherited retinal diseases, assuming the therapeutic gene is expressed appropriately. Currently, a number of PR genes of therapeutic potential have been identified experimentally by virtue of their involvement in recessive human retinal disease and by their ability at least to delay the course of recessive RP-like disease in animal models. These include PDE-β (7, 30) and peripherin/rds (31). Also included in this list should be more general cell survival-promoting factors such as bcl-2 (32) and a variety of growth factors and neurotrophins (33), all of which also have been shown to prolong PR survival in either genetic or environmentally induced animal models of inherited or age-related retinal degeneration (34). Thus, all forms of RP (35) described to date are potential candidates for therapy in this context.
rAAV Mediates Transduction of Postmitotic Cells.
rAAV vectors have been reported to efficiently transduce central nervous system (16), lung (36, 37), and muscle (38, 39), all composed primarily of nondividing cells. We report here equivalently efficient transduction of rodent PRs by using a rod-specific promoter. Although a recent report (40) describes using a similar opsin promoter in a recombinant retrovirus for ex vivo transduction of cultured cells, dissociated retinal cells, and fetal mouse retinal explants, the efficiency was very low (ca. <0.1%), possibly for reasons related to the inefficiency of retroviral vectors to transduce nondividing cells. It is likely, therefore, that the combination of the PR-specific promoter and its packaging in rAAV primarily account for the currently observed high transduction efficiency. The precise mechanism allowing such rAAV transduction, however, has been recently questioned because, in the presence of wtAAv or E4 adenovirus gene expression, rAAV transduction efficiency is greatly enhanced (19, 41). This is apparently due to the increased efficiency of second-strand AAV DNA synthesis, an obligatory initial step in expressing AAV-vectored genes. Additionally, in cells coinfected with either wtAAV or rAAV in the presence of adenovirus, AAV is found at intracellular sites of adenovirus DNA synthesis, again suggesting a role for adenovirus coinfection in efficient rAAV transduction (18). For these reasons, the gfp-containing rAAV was additionally purified on two CsCl gradients and then quantitatively assayed for both helper adenovirus infectious particles and wtAAV (Table 1). This virus preparation contained no detectable infectious helper adenovirus or wtAAV, nor could we detect any non-AAV capsid protein on silver-stained gels (not shown). We conclude, for PRs at least, that rAAV-mediated transduction of nondividing cells in vivo can be an efficient process in the absence of infectious adenovirus or wtAAV.
It is interesting that all test animals exhibited strong PR GFP fluorescence at 6 or 8 weeks postinjection, whereas at 1–3 weeks postinjection, PR fluorescence was weak and often below detection limits. This slow onset of passenger gene expression contrasts to the more rapid (1- to 2-day) detection of GFP signal we reported previously for tissue culture cells (22). This delay in expression may be due to a slow conversion of input rAAV DNA to a transcriptionally active, double-stranded form in nondividing PRs. Without adenovirus gene products to enhance cellular DNA synthetic activities, second-strand AAV DNA synthesis must rely on normal repair synthesis pre-existing in PRs, a process that may only inefficiently produce transcriptionally competent rAAV DNA. This is also consistent with the report of Krishna et al. (19), who found marked enhancement in rAAV transduction of mouse liver and lung 1–2 days after infection with rAAV only if adenovirus was present. The precise physical form of the rAAV DNA in PR cells, whether linear or circular, free or integrated, remains to be determined. Answers at this level will bear on questions of persistence mechanisms and how the limited opsin promoter used is able to achieve the high transduction levels observed.
Subretinal injection detaches the PRs from the RPE and suggests that PRs initially contacting the virus may be responding to the injury of physical separation from the RPE and hence be more responsive to viral transduction. This increase in transduction efficiency has been noted in other tissues (21). Unfortunately, the status of detached PRs with regard to the expression of genes involved in cellular damage control has not been well studied, but initial experiments find evidence for induced gene activity (S. K. Fisher, personal communication). Thus, the current subretinal route of inoculation for efficiently transducing PRs using an opsin promoter also may predispose those cells to more efficiently express AAV-vectored passenger genes, a situation favoring its further exploitation as a potential route toward retinal therapy.
Acknowledgments
We thank Dr. Janis Lem for providing the initial murine rod opsin promoter clone, and Douglas Yasumura, Dr. Michael T. Matthes, Vince Chiodo, Sanford Boye, Weihong Huang, Joan Kiely, Patricia Oh, and Michelle Mo for technical assistance. We thank Drs. Roy H. Steinberg, Adriana Di Polo, Alfred Lewin, John Guy, and Sheldon Miller for helpful comments and discussions on the manuscript. This work was supported by National Institutes of Health Grants EY07864 (W.W.H.), GM53723 (N.M.), EY11123 (W.W.H., J.G.F., N.M.), EY08980 (J.G.F.), EY01919 and EY06842 (M.M.L.); Research to Prevent Blindness, including a Career Development Award (J.G.F,), That Man May See, Inc., and the Foundation Fighting Blindness, Inc.
ABBREVIATIONS
- PR
photoreceptor
- RP
retinitis pigmentosa
- AAV
adeno-associated virus
- rAAV
recombinant AAV
- wtAAV
wild-type AAV
- RPE
retinal pigment epithelium
- GFP
green fluorescent protein
- RT-PCR
reverse transcriptase–PCR
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
References
- 1.Dryja T P. Eye. 1992;6:1–10. doi: 10.1038/eye.1992.2. [DOI] [PubMed] [Google Scholar]
- 2.Bird A C. Am J Ophthalmol. 1995;119:543–562. doi: 10.1016/s0002-9394(14)70212-0. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld P, Dryja T. In: Molecular Genetics of Ocular Disease. Wiggs J L, editor. New York: Wiley–Liss; 1995. pp. 99–126. [Google Scholar]
- 4.Flannery J, Farber D B, Bird A, Bok D. Invest Ophthalmol Visual Sci. 1989;30:191–211. [PubMed] [Google Scholar]
- 5.Dryja T P, Finn J T, Peng Y-W, McGee T L, Berson E L, Yau K-W. Proc Natl Acad Sci USA. 1995;92:10177–10181. doi: 10.1073/pnas.92.22.10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett J, Wilson J, Sun D X, Forbes B, Maguire A. Invest Ophthalmol Visual Sci. 1994;35:2535–2542. [PubMed] [Google Scholar]
- 7.Bennett J, Tanabe T, Sun D, Zeng Y, Kjeldbye H, Gouras P, Maguire A M. Nat Med. 1996;2:649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- 8.Ali R, Reichel M, Thrasher A, Levinsky R, Kinon C, Kanuga N, Hunt D M, Bhattacharya S. Hum Mol Genet. 1996;5:591–594. doi: 10.1093/hmg/5.5.591. [DOI] [PubMed] [Google Scholar]
- 9.Li T, Adamian M, Roof D, Berson E, Dryja T, Roessler B, Davidson B. Invest Ophthalmol Visual Sci. 1994;35:2543–2549. [PubMed] [Google Scholar]
- 10.Mashhour B, Couton D, Perricaudet M, Briand P. Gene Therapy. 1994;1:122–126. [PubMed] [Google Scholar]
- 11.Lem J, Applebury M, Falk J, Flannery J, Simon M. Neuron. 1991;6:201–210. doi: 10.1016/0896-6273(91)90356-5. [DOI] [PubMed] [Google Scholar]
- 12.Zack D, Bennett J, Wang Y, Davenport C, Klaunberg B, Gearhart J, Nathans J. Neuron. 1991;6:187–199. doi: 10.1016/0896-6273(91)90355-4. [DOI] [PubMed] [Google Scholar]
- 13.Morabito M, Yu X, Barnstable C. J Biol Chem. 1991;266:9667–9672. [PubMed] [Google Scholar]
- 14.DesJardin L, Hauswirth W. Invest Ophthalmol Visual Sci. 1996;37:154–165. [PubMed] [Google Scholar]
- 15.Muzyczka N. Curr Top Micro Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 16.Kaplitt M, Leone P, Samulski R, Xiao X, Pfaff D, O’Malley K, During M. Nat Genet. 1993;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 17.Berns K, Muzyczka N, Hauswirth W. In: Virology. Fields B, editor. New York: Raven; 1985. pp. 415–432. [Google Scholar]
- 18.Weitzman M, Fisher F, Wilson J. J Virol. 1996;70:1845–1854. doi: 10.1128/jvi.70.3.1845-1854.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishna F, Gao G, Weitzman M, DeMatteo R, Burda J F, Wilson J. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell D W, Alexander I E, Miller A D. Proc Natl Acad Sci USA. 1995;92:5719–5723. doi: 10.1073/pnas.92.12.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell D W, Miller A D, Alexander I E. Proc Natl Acad Sci USA. 1994;91:8915–8919. doi: 10.1073/pnas.91.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zolotukhin S, Potter M, Hauswirth W, Guy J, Muzyczka N. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaughlin S, Collis P, Hermonat P, Muzyczka N. J Virol. 1988;62:1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanes J, Rubenstein J, Nicolas J. EMBO J. 1986;5:3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasband W, Bright D. Microbeam Anal Soc J. 1995;4:137–149. [Google Scholar]
- 26.van Ginkel P, Hauswirth W. J Biol Chem. 1994;269:4986–4992. [PubMed] [Google Scholar]
- 27.Farber D, Flannery J G, Lolley R, Bok D. Invest Ophthalmol Visual Sci. 1981;20:24–31. [PubMed] [Google Scholar]
- 28.Curcio C, Sloan K, Kalina R, Hendrickson A. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 29.Osterberg G. Acta Ophthalmol. 1935;6:1–103. [Google Scholar]
- 30.Lem J, Flannery J, Li T, Applebury M, Farber D, Simon M. Proc Natl Acad Sci USA. 1992;89:4422–4426. doi: 10.1073/pnas.89.10.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Travis G H, Groshan K R, Lloyd M, Bok D. Neuron. 1992;33:113–119. doi: 10.1016/0896-6273(92)90226-4. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Flannery J G, LaVail M M, Steinberg R H, Xu J, Simon M I. Proc Natl Acad Sci USA. 1996;93:7042–7047. doi: 10.1073/pnas.93.14.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faktorovitch E, Steinberg R H, Yasumura D, Matthes M, LaVail M M. Nature (London) 1990;347:83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- 34.Steinberg R H. Curr Opin Neurobiol. 1994;4:515–524. doi: 10.1016/0959-4388(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 35.Daiger S, Sullivan L, Rodriguez J. Behav Brain Sci. 1995;18:452–467. [Google Scholar]
- 36.Afione S, Conrad C, Kearns W, Chunduru S, Adams R, Reynolds T, Guggino W, Cutting G, Carter B, Flotte T. J Virol. 1996;70:3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flotte T, Afione S A, Conrad C, McGrath SA, Solow R, Oka H, Zeitlin P L, Guggino W B, Carter B J. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao X, Li J, Samulski R. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kessler P, Podsakoff G, Chen X, McQuiston S, Colosi P, Matelis L, Kurtzman G, Byrne B. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kido M, Rich K, Lang G, Barron E, Kohn D, Al-Ubaidi M, Blanks J. Curr Eye Res. 1996;15:333–344. doi: 10.3109/02713689609017624. [DOI] [PubMed] [Google Scholar]
- 41.Ferrari F, Samulski T, Shenk T, Samulski R. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]