Abstract
In mammalian cells, cyclin E–CDK2 complexes are activated in the late G1 phase of the cell cycle and are believed to have an essential role in promoting S-phase entry. We have targeted the murine genes CCNE1 and CCNE2, encoding cyclins E1 and E2. Whereas single knockout mice were viable, double knockout embryos died around midgestation. Strikingly, however, these embryos showed no overt defects in cell proliferation. Instead, we observed developmental phenotypes consistent with placental dysfunction. Mutant placentas had an overall normal structure, but the nuclei of trophoblast giant cells, which normally undergo endoreplication and reach elevated ploidies, showed a marked reduction in DNA content. We derived trophoblast stem cells from double knockout E3.5 blastocysts. These cells retained the ability to differentiate into giant cells in vitro, but were unable to undergo multiple rounds of DNA synthesis, demonstrating that the lack of endoreplication was a cell-autonomous defect. Thus, during embryonic development, the needs for E-type cyclins can be overcome in mitotic cycles but not in endoreplicating cells.
Keywords: cell cycle/cyclin E/endoreplication/placenta/trophoblast
Introduction
Cyclin-dependent kinases (CDKs) orchestrate progression through the cell cycle in all eukaryotes. Activation of CDKs depends upon association with their regulatory subunits, the cyclins, in specific pair-wise combinations. In vertebrates, two types of CDKs are active through the initial (G1) phase of the cell cycle: the first includes CDK4 and CDK6, associated with D-type cyclins (e.g. Matsushime et al., 1992); the second is CDK2, associated with cyclin E (e.g. Dulic et al., 1992; Koff et al., 1992) and, upon S-phase entry, with cyclin A. Expression of D-type cyclins depends upon extracellular mitogens, whereas that of cyclins E and A displays an intrinsic cyclic behavior (for reviews, see Sherr and Roberts, 1999).
Both types of CDK complexes target the Retinoblastoma-family or ‘pocket’ proteins pRb, p107 and p130. These proteins negatively regulate cell cycle progression, mainly by binding to the E2F-family transcription factors. The function of cyclin D–CDK4/6 is dispensable in cells defective for either pRb alone (Guan et al., 1994; Lukas et al., 1995; Medema et al., 1995) or p107 and p130 together (Bruce et al., 2000). Similarly, binding of pRb proteins by the adenoviral oncoprotein E1A renders cells insensitive to the CDK4/6 inhibitor p16INK4a (Alevizopoulos et al., 1998). Thus, pocket proteins appear to be the essential substrates of cyclin D–CDK complexes. Cyclin E cooperates with D-type cyclins in achieving full phosphorylation and inactivation of pRb and p130, but not p107 (Beijersbergen et al., 1995; Xiao et al., 1996; Lundberg and Weinberg, 1998; Harbour et al., 1999). Unlike D-type cyclins, however, cyclin E appears to be required for S-phase entry in pRb-null cells (Ohtsubo et al., 1995) and can promote G1–S progression independently from pRb and p130 phosphorylation (Alevizopoulos et al., 1997; Lukas et al., 1997; Kelly et al., 1998). In addition, in order to bypass cell cycle arrest by the CDK2 inhibitor p27Kip1, E1A requires a second function besides pRb binding (Alevizopoulos et al., 1998). Collectively, these observations pointed to pRb-independent function(s) of cyclin E–CDK2 in G1–S progression. Cyclin E–CDK2 phosphorylates two of its own regulators, CDC25A (Hoffmann et al., 1994) and p27Kip1 (Sheaff et al., 1997; Vlach et al., 1997; see Discussion), suggesting positive feedback mechanisms. Other possible substrates include proteins involved in histone gene expression (NPAT: Ma et al., 2000; Zhao et al., 2000), centrosome duplication (nucleophosmin: Okuda et al., 2000), chromatin modifications (p300/CBP: Ait-Si-Ali et al., 1998; SWI/SNF: Shanahan et al., 1999), pre-mRNA splicing (Seghezzi et al., 1998) and DNA replication (for review, see Ewen, 2000). In vitro studies in mammalian and Xenopus cell-free systems have provided strong evidence for a direct role of cyclin E–CDK2 in regulating the initiation of DNA replication (Jackson et al., 1995; Krude et al., 1997; Furstenthal et al., 2001a,b; Coverley et al., 2002).
In contrast with cyclin E (hereafter referred to as E1), very few studies have directly addressed the function of cyclin E2. These two cyclins are closely related and display very similar cell cycle-regulated expression and biochemical properties, in particular activation of CDK2 and inhibition by p27 (Lauper et al., 1998; Zariwala et al., 1998; Gudas et al., 1999). Furthermore, the respective mRNAs are expressed in an overlapping manner during mouse embryonic development (Geng et al., 2001). To address the function of E-type cyclins, we knocked out their genes in the mouse. We report that these cyclins are redundant during development and have an essential function in endoreplication of placental giant trophoblast cells, but not in embryonic cell cycles.
Results
Disrupting the cyclin E genes
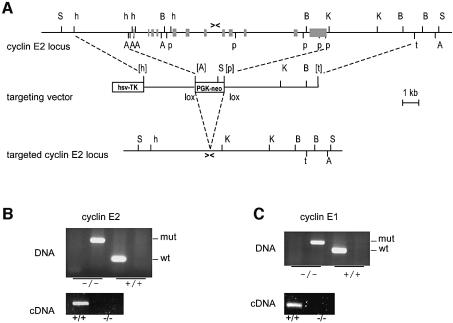
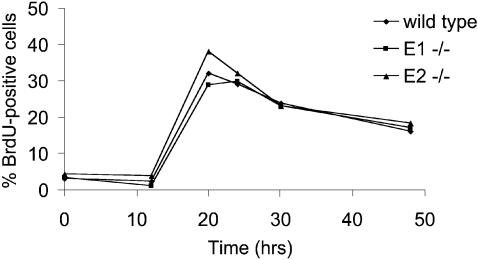
To disrupt the murine cyclin E2 gene (CCNE2), genomic sequences spanning the 5′ UTR, the coding region and part of the 3′ UTR, were replaced by a neo cassette (Figure 1A). After electroporation of the targeting vector and G418 selection, correctly targeted cyclin E2+/– (129Sv) embryonic stem (ES) cells were identified by Southern blotting (data not shown). Selected clones were transiently transfected with a CRE expression vector to eliminate neo and subsequently injected in C56Bl/6 blastocysts, generating chimera that transmitted the mutated allele through the germline. The resulting cyclin E2+/– progeny in 129Sv/C56Bl/6 mixed background were then intercrossed, genotyped by PCR (Figure 1B, upper) and tested by reverse transcription and PCR for the absence of cyclin E2 mRNA (Figure 1B, lower). Viable cyclin E2-null mice were observed at a frequency (40/150) consistent with the expected Mendelian ratio (1/4) and exhibited no measurable growth impairment. Mutant females were fertile, whereas males exhibited partial sterility. Histological analysis of 2-month-old cyclin E2–/– males revealed that their testes were about two-thirds of the normal size, and the number of mature spermatozoa was reduced by 25–70%. In parallel with cyclin E2, we generated cyclin E1 mutant mice, starting with recombinant cyclin E1+/– ES cells bearing a neo cassette in place of the entire CCNE1 coding region (a gift from Peter Sicinski; Geng et al., 2003). Genotyping and expression analysis of offspring (Figure 1C) revealed that viable cyclin E1-null mice were present at the expected frequency (52/216), developed normally and were fully fertile. To test whether cells lacking either cyclin E2 or E1 had measurable defects in proliferation, we isolated mouse embryonic fibroblasts (MEFs) from E13.5 embryos. MEF populations of either mutant genotype grew out as efficiently as wild-type control MEFs (data not shown) and retained a normal capacity to re-enter the cell cycle from quiescence (Figure 2). Altogether, the above results show that cyclins E1 and E2, taken individually, are dispensable for normal development and cell proliferation.
Fig. 1. Targeted disruption of CCNE2 in mice. (A) Schematic representation of the CCNE2 locus (top), targeting vector (middle) and targeted allele (bottom). Gray boxes represent the exons. The arrowheads represent the PCR primers used to amplify the wild-type and targeted alleles for genotyping. Restriction enzymes: A, ApaI; t, AatI; B, BamHI; h, SphI; K, KpnI; p, SpeI; S, SacI. The sites in brackets were used for cloning but lost in the process. See Materials and methods for experimental details. (B and C) PCR-based genotyping (top) and mRNA analysis (bottom) from CCNE2 and CCNE1 mutants, respectively. Genomic DNA was extracted from ear-punches and RNA from E13.5 mouse embryonic fibroblasts.
Fig. 2. Cyclin E1–/– and E2–/– single knockout cells are not impaired in cell cycle entry. The indicated wild-type and mutant mouse embryonic fibroblasts (from two independent preparations) were synchronized by serum starvation and contact inhibition, and subsequently released into cell cycle. DNA synthesis was monitored by flow cytometric analysis of DNA content and bromodeoxyuridine incorporation.
To determine whether E-type cyclins were redundant, we generated the cyclin E1–/–E2–/– double knockout (dKO). Crosses of the single-mutant strains yielded E1+/–E2+/– animals that were subsequently intercrossed. Of 189 newborns genotyped, none was a dKO (expected 1/16, or ≥11 mice). All other genotypes were viable and observed at Mendelian ratios. In particular, E1+/–E2–/– and E1–/–E2+/– animals developed like the respective single-mutant controls (hence, E1+/–E2–/– males were partially sterile, like E2–/– males). To increase the frequency of the dKO genotype, subsequent breedings were derived from E1–/–E2+/– (of both sexes) and E1+/–E2–/– animals (females only). Of >400 newborns genotyped, none was a dKO (expected 1/4, or ≥100 mice). Thus, at least one E-type cyclin allele is required and sufficient for survival.
Mice lacking both cyclin E1 and E2 die from causes unrelated to cell proliferation
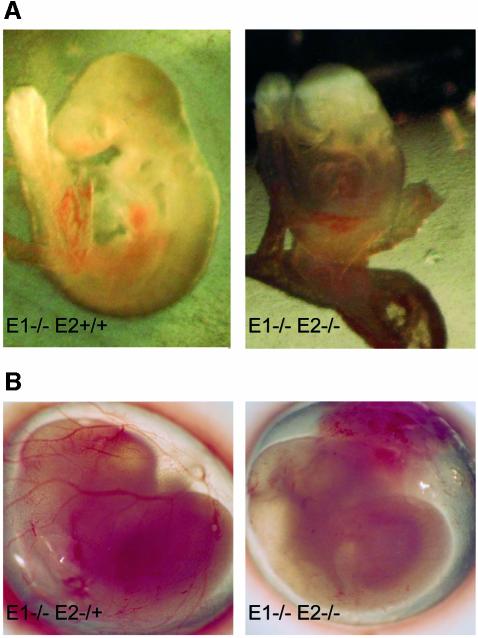
To determine the stage of lethality, we collected and genotyped embryos at various stages of development. At E11.5, dKO embryos were recovered at the expected Mendelian ratio (10/39) but most were dead, with only 2/10 showing a beating heart. At E10.5, dKO embryos were normally represented (16/73) and most of them were still alive. Thus, lethality occurred mainly between E10.5 and E11.5. The morphology of dKO embryos between E9.5 and E11.5 presented no obvious defects, but instead manifested a general developmental delay. dKO embryos were, with few exceptions, smaller and paler than their siblings (Figure 3A) and, in 60% of them, the yolk sac at E11.5 was poorly vascularized (Figure 3B). Furthermore, two E10.5 dKO embryos analyzed by whole mount staining with α-PECAM antibodies showed an arrangement of cranial vasculature typical of E9.5 embryos (data not shown). Altogether, these data suggest that dKO conceptuses (embryo and extra-embryonic tissues) suffer from general growth retardation and defects in vascularization.
Fig. 3. Phenotypes of cyclin E1–/–E2–/– double knockout (dKO) embryos. (A) E10.5 dKO embryos are smaller than control siblings. This relative difference in size was observed in different litters. (B) The vasculature in the dKO yolk sac at E11.5 is nearly absent. Note that the characteristic pattern of yolk sac vasculature in the control (left) is missing in the dKO (right).
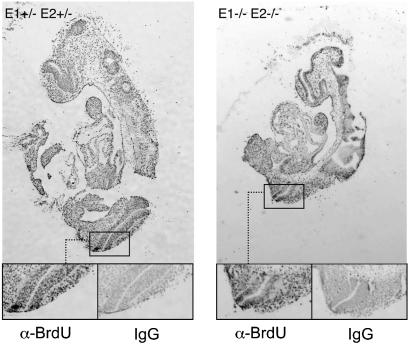
To address whether cell proliferation was impaired in dKO embryos, we measured DNA synthesis in situ by bromodeoxyuridine (BrdU) staining of sections at E9.5–E11.5. Surprisingly, dKO embryos and their siblings showed no evident differences (Figure 4). Altogether, eight mutant embryos and matched controls were examined at different stages, with identical results (data not shown). This finding suggests that compensatory mechanisms operate in dKO cells to allow cell cycle progression (see Discussion). Understanding these mechanisms will require tractable cells, such as E13.5 MEFs. We have attempted to derive dKO cells at earlier stages, but have obtained very heterogeneous populations. Nonetheless, loss of E-type cyclins in dKO embryos causes no overt defects in cell proliferation at the time of lethality.
Fig. 4. Bromodeoxyuridine staining of E10.5 embryos shows equally abundant S phases in double knockout and control siblings.
Requirement of cyclins E1 and E2 for endoreplication in trophoblast giant cells
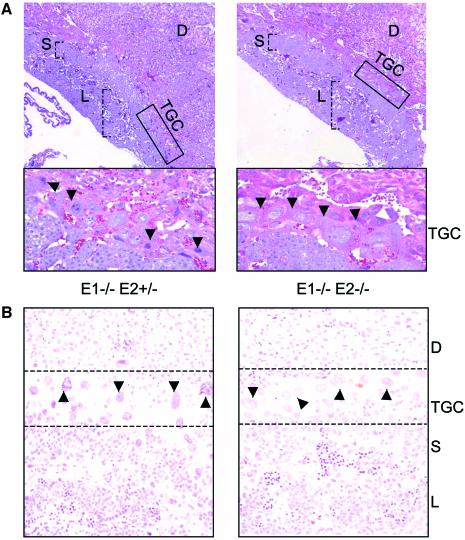
There are various genes whose absence during development leads to extra-embryonic defects (e.g. Luo et al., 1997; Kraut et al., 1998), and for some of them the involvement in placental formation has been unexpected (Schorpp-Kistner et al., 1999; Tremblay et al., 2001). For instance, two recent papers describe extra-embryonic defects in mice lacking the cell cycle regulators DP1 and pRb (Kohn et al., 2003; Wu et al., 2003). Placental defects cause poor exchange of metabolites and oxygen, leading to secondary phenotypes such as developmental delay and yolk sac abnormalities, as seen in our dKO conceptuses. Histological analysis on sections of placentas stained with haemotoxylin and eosin (H&E) at stages E10.5 and E11.5 revealed a clear paucity in the nuclear DNA content of trophoblast giant cells (TGCs; Figure 5A), which was confirmed by quantitative Feulgen staining of DNA (Figure 5B). During differentiation of trophoblast stem cells (TSCs) into TGCs, multiple rounds of DNA synthesis occur without mitotic division (endoreplication) leading to increases in DNA content up to 1000N and to a parallel increase in nuclear size (Barlow and Sherman, 1972; Varmuza et al., 1988; Zybina and Zybina, 1996). TGCs are believed to be involved in the remodeling of the maternal uterus at implantation and, at later stages, for production of hormones and cytokines necessary for the maintenance of a proper embryo–mother interface (Cross et al., 1994; Cross, 2000). These key functions in placental development made TGCs prime candidates for the lethality of dKO embryos. Thus, we decided to address whether a decrease in endoreplication was a primary, cell-intrinsic defect of TGCs lacking E-type cyclins.
Fig. 5. Trophoblast giant cells in double knockout (dKO) placentas have a reduced amount of DNA. Haemotoxylin and eosin (A) and Feulgen (B) staining of sections through E10.5 placentas. The giant cell layer is either magnified in the inserts (A) or indicated by the dotted lines (B). The arrowheads show the giant nuclei: note the intense staining of nuclei in the control embryos, which is proportional to DNA content and is missing in the dKO. The placental layers are indicated as follows: D, decidua; TGC, trophoblast giant cells; S, spongiotrophoblast; L, labyrinth.
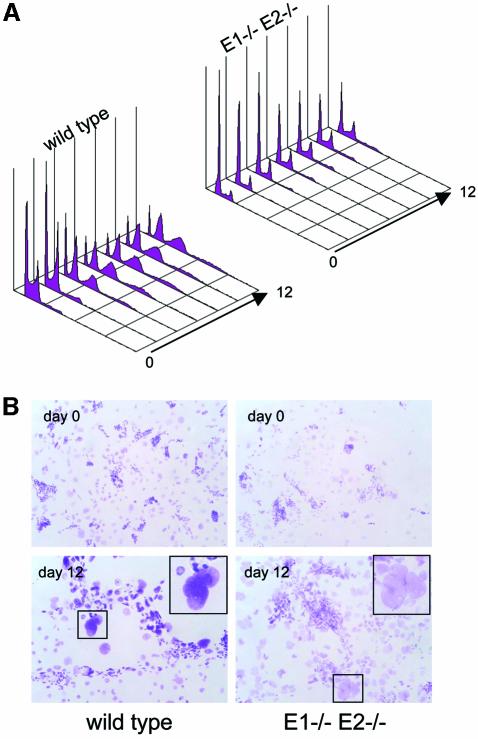
To achieve this goal, we derived TSCs from dKO and wild-type blastocysts (Tanaka et al., 1998). TSCs in the blastocyst constitute the polar trophoectoderm, maintained in an undifferentiated state by FGF4 produced by the underlying inner cell mass. TSCs are the precursors of the extra-embryonic ectoderm and the ectoplacental cone, which subsequently gives rise to the secondary giant cells, the labyrinth and spongiotrophoblast layers. When starved of FGF4, heparin and MEF-conditioned medium (MCM), TSCs give rise to all the trophoblast lineages in vitro, recapitulating in vivo placental processes (Tanaka et al., 1998). The trophoblasts committed to the TGC lineage adhere to the plate more tightly than the other cells and can be enriched by light trypsinization (Cross et al., 1995). Following this treatment, we initiated differentiation and allowed it to proceed for a total of 12 days, harvesting cells every 2 days. Analysis of DNA content, performed by flow cytometry (Figure 6A) or Feulgen staining (Figure 6B) gave striking results: unlike wild-type TSCs, dKO TSCs were unable to undergo multiple rounds of DNA replication. Only a few mutant cells reached a ploidy of 8N, whereas a large fraction of wild-type cells reached 8N and more (Figure 6A). dKO cells had not increased their DNA content after 26 days (data not shown), indicating they were not simply retarded in endoreplication and/or differentiation (see below).
Fig. 6. Cyclins E1 and E2 are required for endoreplication in trophoblast giant cells (TGCs) in vitro. (A) Flow cytometric analysis of DNA content in nuclei of differentiating trophoblast stem cells (TSCs). (B) Feulgen staining of undifferentiated TSCs (day 0) and following differentiation into TGCs (day 12).
Uncoupling TGC differentiation from endoreplication
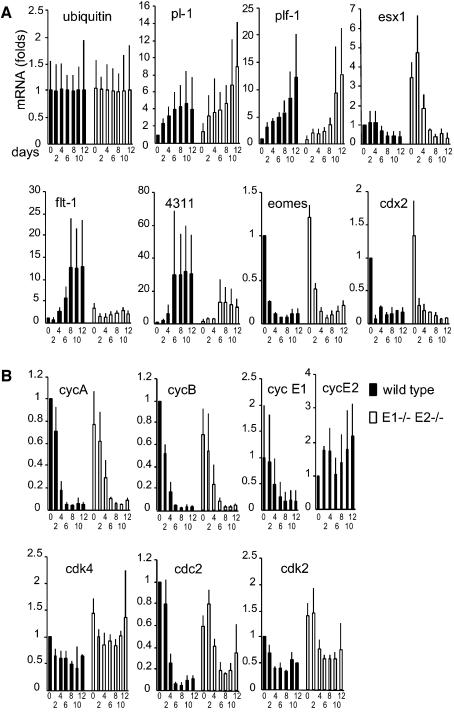
Surprisingly, in spite of the differences in DNA content, the nuclei of wild-type and mutant TGCs reached similar sizes in vitro (Figure 6B) as well as in vivo (Figure 5), suggesting that these two parameters are not interdependent. The overall morphology of wild-type and dKO giant cells also appeared comparable (data not shown). These observations suggested that differentiation proceeded in mutant TSCs, in spite of the block in endoreplication. This concept was further supported by mRNA expression analysis, conducted by real-time PCR, for several differentiation markers characteristic of the different trophoblast cell lineages. Markers of TGC differentiation such as pl-1 (placental lactogen-1) and plf-1/proliferin (Lee et al., 1988; Faria et al., 1991) were similarly induced in wild-type and dKO cells (Figure 7A). Esx-1, a marker of the labyrinthine layer (Yan et al., 2000), showed elevated expression in dKO cells at early, but not later, time-points. Conversely, the spongiotrophoblast markers 4311 (tpbp) and flt-1 (Lescisin et al., 1988; Dumont et al., 1995) showed lower expression in dKO cells, suggesting that there may be defects in this lineage. Two markers of undifferentiated TSCs, eomes and cdx2 (Beck et al., 1995; Russ et al., 2000), were similarly downregulated in wild-type and mutant cells, confirming that differentiation was not blocked altogether.
Fig. 7. mRNA expression analysis in differentiating trophoblast stem cells (TSCs). Wild-type and double knockout TSCs were induced to differentiate for 12 days. Every 2 days, RNA was isolated and expression of specific mRNAs was quantified with reverse transcription and real-time PCR. The results, normalized to ubiquitin, are expressed as fold-induction relative to day 0 in the wild-type, and represent the average of 3–5 different experiments from different TSC clones. (A) Ubiquitin mRNA (control) and placental markers. (B) Cell cycle genes.
Genes associated with progression through the mitotic cycle, such as CDC2 and cyclins A and B were downregulated upon differentiation in both wild-type and dKO cells (Figure 7B). CDK4 and CDK2 mRNAs were expressed at relatively constant levels in cells of both genotypes. CDK2 protein levels and CDK2-associated kinase activity were also retained in the mutant (data not shown), presumably due to association with cyclin A, which constitutes the bulk of CDK2 activity. The mRNAs for cyclins E1 and E2 showed a different pattern of regulation during TSC differentiation: E1 was downregulated (although still expressed at significant levels at day 12), whereas E2 mRNA levels remained constant.
Discussion
E-type cyclins are required for endoreplication in trophoblasts, but not for mitotic cycles in the mouse embryo
The deletion of a key cell cycle regulator would be expected to halt cell proliferation and block development at early stages, coincident with the depletion of maternal stores. For example, embryos lacking Max (the essential partner of Myc proteins) or cyclin A2 (the only A-type cyclin expressed in somatic cells) die shortly after implantation (E5.5–E6.5; Murphy et al., 1997; Shen-Li et al., 2000). As shown in this paper and in a parallel study (Geng et al., 2003), deletion of E-type cyclins yielded a dramatically different result. Cyclin E1–/–E2–/– (dKO) embryos developed until midgestation (E10.5) and did not display any overt defect in cell proliferation. Furthermore, animals lacking the cyclin E and A partner CDK2 developed normally into adulthood (Ortega et al., 2003). Studies of cultured cell lines had already suggested that cyclin E–CDK2 activity could be dispensable for cell proliferation under certain conditions (Alevizopoulos et al., 1998; Tetsu and McCormick, 2003), but did not allow us to anticipate that normal development could occur without it. Although the new genetic data would suggest that cyclin E and CDK2 control no essential cell cycle transition, we deem it more likely that they do, but can be compensated for during development. We speculate that other components of the cell cycle machinery, such as cyclin A–CDK2, cyclin A–CDC2, cyclin D–CDK4, cyclin B–CDC2 or others are able to substitute in the different knockouts. In favor of such plasticity, mitotic cyclin B–CDC2 complexes have the potential to induce S-phase entry if targeted to the nucleus (Moore et al., 2003) and loss of two out of the three D-type cyclins can be compensated spatially and temporally in a developing mouse (Ciemerych et al., 2002).
Cyclin E1–/–E2–/– (dKO) conceptuses showed developmental delays consistent with a placental dysfunction (see Results). At the histological level, the only defect that we detected in dKO placentas was a paucity of nuclear DNA staining in TGCs (trophoblast giant cells). By deriving TSCs (trophoblast stem cells) from dKO and wild-type blastocysts (E3.5), we demonstrated that the lack of endoreplication is an intrinsinc, cell-autonomous defect of dKO cells, whereas the TGC differentiation program per se is not blocked. In a parallel study, Geng et al. (2003) derived dKO ES cells and used the technique of tetraploid complementation to obtain genetic evidence that the lethality of cyclin E1–/–E2–/– embryos derives from a placental defect. Altogether, these data suggest that the endoreplicative defect of cyclin E1–/–E2–/– TGCs is a direct cause of embryonic lethality.
In Drosophila, cyclin E functions in both endoreplicative and mitotic cycles (Knoblich et al., 1994; Duronio and O’Farrell, 1995; Sauer et al., 1995; Follette et al., 1998). It is therefore likely that a similar function is required during both types of cycles in vertebrates (see Introduction; MacAuley et al., 1998). One process that appears to be directly regulated by cyclin E in vertebrates is the initiation of DNA replication (Jackson et al., 1995; Krude et al., 1997; Furstenthal et al., 2001a,b; Coverley et al., 2002). In this regard, an important difference may exist between proliferating cells (i.e. that undergo successive mitotic cycles) and cells that re-enter the mitotic cycle after quiescence (or G0). An elegant series of in vitro studies (Coverley et al., 2002) suggested that cyclin A alone may suffice to drive DNA synthesis in cycling cells, whereas S-phase entry from G0 requires cyclin E in order to promote the formation of pre-replication complexes. Periodic (as opposed to continuous) expression of cyclin E is required for sustained endoreplication in Drosophila (Follette et al., 1998) and is also observed in rat TGCs (MacAuley et al., 1998). Furthermore, in both endoreplicative and mitotic cycles in Drosophila, cyclin E is required for the loading of mcm proteins onto replication origins (Su and O’Farrell, 1997, 1998). Thus, S-phase initiation in endoreduplicating cells may have requirements similar to those in cells that re-enter the cycle. Consistent with this hypothesis, E13.5 MEFs derived from rescued cyclin E1–/–E2–/– mouse embryos displayed a selective defect in cell cycle re-entry (Geng et al., 2003). CDK2, instead, was dispensable for S-phase entry from G0 and, by definition, for endoreplication, since mutant mice were viable (Ortega et al., 2003). In summary, mouse genetics indicate a requirement for cyclin E (as opposed to CDK2) in endoreplication and cell cycle re-entry, perhaps through a common biochemical mechanism. The fact that development of cyclin E1–/–E2–/– embryos occurs until midgestation when they die due to placenta failure, and can even be rescued until birth (Geng et al., 2003), implies that cell cycle re-entry from quiescence is not critical during embryogenesis.
How do we reconcile mouse genetics with cell cycle textbooks?
Altogether, our data and the two parallel studies (Geng et al., 2003; Ortega et al., 2003) establish that cyclin E (i.e. E1 and E2) and CDK2 are dispensable for cell proliferation in the mouse. This contrasts with a large body of literature indicating that cyclin E–CDK2 is required for G1–S progression (see Introduction). Another fundamental puzzle is raised by the difference in phenotypes between CDK2 and cyclin E1/E2 knockout mice, since only the latter die of placental defects. We speculate that a unique mechanism exists that will resolve these apparent discrepancies, and provide here a working hypothesis.
Mammalian cells that are growth-arrested almost invariably contain ternary complexes in which cyclin E–CDK2 is inhibited by the CKIs p21 or p27, whereas cyclin A is downregulated (e.g. Polyak et al., 1994; Sherr and Roberts, 1999). Ectopic expression of p21 or p27 reiterates this situation (e.g. Vlach et al., 1996, 1997; Sheaff et al., 1997). The CKIs possess distinct contact sites for cyclin E (or A) and CDK2, both of which are required for ternary complex formation and cell cycle arrest (e.g. Russo et al., 1996; Vlach et al., 1997). Thus, association of CKIs with cyclin E–CDK2 appears to be critical for cell cycle arrest.
We propose that the dominant-inhibitory signal in cell cycle progression is the formation of the ternary cyclin E–CDK2–p27 (or p21) complex itself, rather than the mere loss of CDK2 catalytic activity. In this context, CDK2 kinase activity must be required to ensure that p27 is degraded in a timely manner. Indeed, one function of cyclin E–CDK2 is to phosphorylate p27, inducing its ubiquitination and degradation (Sheaff et al., 1997; Vlach et al., 1997; Montagnoli et al., 1999). Additional, phosphorylation-independent mechanisms exist to degrade p27 (Malek et al., 2001), but these may also be triggered by cyclin E–CDK2 (Furstenthal et al., 2001b). The critical difference between proliferating and quiescent (or endoreplicating) cells might be that the latter accumulate p27 (and hence ternary complexes) above a critical inhibitory threshold. In other words, continuously proliferating cells may not require cyclin E-induced degradation of p27.
This scenario would also account for the phenotypic differences between cyclin E1/E2- and CDK2-deleted mice. In cells lacking E-type cyclins, cyclin A–CDK2 is expected to be present. Cyclin A also forms inactive ternary complexes with CDK2 and p27 (e.g. Russo et al., 1996) but, unlike cyclin E, is unable to trigger p27 degradation (Sheaff et al., 1997; Vlach et al., 1997). In cells lacking CDK2, cyclin E appears to remain uncomplexed, but cyclin A is associated with kinase activity (Ortega et al., 2003). Hence, cyclin A–CDC2 (normally active at G2–M) and/or other p27-resistant complexes might form in late G1. Thus, the differential sensitivity of compensatory complexes to CKIs, including p27, p21 and the related p57Kip2 in trophoblasts (Zhang et al., 1998), might explain why E-type cyclins are required, whereas CDK2 is dispensable for cell cycle re-entry and endoreplication. As a corollary, we predict that cells lacking CDK2, but not those lacking E-type cyclins, will escape p27- or p21-induced arrest. The key feature of this model is that it does not invoke CDK2-independent activities of cyclin E, although these remain a formal possibility.
Contrary to current models (Burdon et al., 2002), the knockout studies also imply that cyclin E and CDK2 are dispensable for proliferation of pluripotent stem cells. In particular, Geng et al. (2003) directly derived ES cells from cyclin E1–/–E2–/– embryos. It remains to be addressed, however, whether cyclin E–CDK2 activity is rate-limiting for the exceptionally rapid cycling of ES cells, as suggested by a study with pharmacological inhibitors (Stead et al., 2002). The same study implied that the peculiar structure of the ES cell cycle, in particular the absence of a proper G1 phase, depends upon regulators other than CDK2. We note that ES cells do not express p21 and p27 (Stead et al., 2002), consistent with our proposal that E-type cyclins are dispensable for cell cycle progression per se in the absence of these CKIs.
If cyclin E–CDK2–p27 complexes arrest the cycle, what are their targets? The sensitive downstream events may include transcription of E2F-target genes (such as cyclin A), the assembly of pre-replicative complexes (Furstenthal et al., 2001a,b) and/or any of the other processes in which cyclin E–CDK2 has been attributed a regulatory role (see Introduction). In certain tumor lines (Tetsu and McCormick, 2003) or upon expression of adenovirus E1A (Alevizopoulos et al., 1998), bypass mechanisms exist that render cells insensitive to inhibition of CDK2 by p27. Understanding these mechanisms is likely to shed new light on the functional targets of cyclin E–CDK2–p27.
Materials and methods
Generation of cyclin E1- and E2-deficient mice
A mouse genomic 129Sv λ GEM-11 library (see Radtke et al., 1999) was screened with a murine CCNE2 cDNA probe. Genomic clones spanning 25 kb of the locus were obtained. To target CCNE2, a 3681 bp SphI–ApaI fragment upstream of the first codon (5′ arm) was cloned in the TNLOX-1-3 vector (Radtke et al., 1999) between the hsv-thymidine kinase cassette and the PGK promoter-neor cassette. A 5511 bp SpeI–AatII genomic fragment (3′ arm) was cloned downstream of PGK-neor (Figure 1A). Both arms are 99% identical to corresponding regions flanking CCNE2 in the annotated sequence of mouse (C57BL/6J) chromosome 4 (NT_039258). The endpoints are GCATGCCATACAC TCGTTTA to CCTCCCGGAGGGGTGGGCCC (5′ arm) and ACTA GTTTGTCTGCCTTGCC to TACTCCAAGATTGTGACGTC (3′ arm). ES cells (129Sv) were electroporated with the linearized targeting construct and selected in G418 (400 µg/ml) and ganciclovir (2 µM). Proper recombination was confirmed by Southern blotting (data not shown) and resulted in deletion of a 12.3 kb genomic sequence spanning the 5′ UTR, all coding exons, as well as part of the 3′ UTR. Finally, transient transfection with a plasmid expressing CRE recombinase resulted in deletion of the neor cassette (Figure 1A). CCNE1-targeted ES cells, in which exons 2–11 were replaced with a neor cassette, were generated in the laboratory of Peter Sicinski (Geng et al., 2003) and kindly provided to us. Either CCNE1- or CCNE1-targeted ES cells were microinjected into C57BL/6 blastocysts. Male chimeras were obtained that transmitted either targeted allele into the germline. Genotypic analysis was performed by PCR on ear punches with the following primers: CCNE1 wild-type allele: CGCCATGGTTATCCGGGAGATGG and CGCATACTGAGACACAGACT; CCNE1 mutant: GATCTCTCG TGGGATCATTG and same as above; CCNE2 wild-type: CAGTGC TCTTTGCAGCTGTATCA and GGATATTAATGTGTTCAACCCC TCA; CCNE2 mutant: CGTCCTCTCCTGTCATTGGC and GAAAT ATGGCAAGGCAGACAAACTA.
Histology and BrdU incorporation
Conceptuses, still within the uterus or dissected at various stages of development, were fixed in 4% paraformaldehyde for 24 h and embedded in Paraplast Plus (Fisher Scientific). Paraffin sections (5 µm) were stained with H&E or Feulgen. For S-phase labeling, BrdU (100 µg/g body weight) was injected intra-peritoneally into pregnant females at 10.5 days post-coitum. The females were killed 2 h after injection. BrdU incorporation in embryos was detected on 5 µm paraffin sections with anti-BrdU antibodies (Becton Dickinson) used in combination with the Vectastatin ABC Kit (Vector Laboratories).
Isolation, culture and analysis of TSCs
Blastocysts were flushed out and collected from pregnant female uteri at E3.5 in M16 medium (Sigma Aldrich). Individual blastocysts were seeded onto mitomycin-treated MEFs, provided with 1 ng/ml FGF-4 and 1 U/ml heparin and cultured at 37°C with 5% CO2. After initial expansion on MEF layers (4–5 passages), TSCs were cultured in 70% MCM and 30% fresh RPMI-1640 Glutamax (Gibco-BRL) supplemented with 1 ng/ml FGF-4 and 1 U/ml heparin (Tanaka et al., 1998). For preparation of MCM, E12.5 MEFs (isolated from CD-1 mice, Charles River Laboratory) were treated with 10 µg/ml mitomycin C for 3 h, trypsinized and replated at confluent density in RPMI-1640 Glutamax supplemented with 20% FBS (ES-cell screened and decomplemented), 50 µg/ml pen-strep, 1 mM sodium pyruvate and 100 µM β-mercaptoethanol. MCM was collected every 3 days for 9 days, filtered and frozen.
TSC differentiation was induced on the second day after passage. Cells were trypsinized for 2 min, and the remaining trypsin-resistant cells were cultured in differentiation medium (growth medium minus MCM, FGF-4 and heparin). For analysis of DNA content, differentiating TSCs (on 6 cm dishes) were harvested every 2 days by trypsinization, washed with PBS and fixed in 70% EtOH. At the end of the differentiation time course (12 days), all collected samples cells were washed with PBS, fixed again with 1% formaldeyde, washed in PBS and treated with 0.08% pepsin in 0.1 N HCl for 20 min at 37°C. The resulting nuclei were washed once in 0.1 M Na-borate pH 8.0 and once in IFA (10 mM HEPES pH 7.4, 150 mM NaCl, 4% FCS, 0.1% sodium azide) supplemented with 0.5% Tween-20, resuspended in 1 ml of IFA containing 0.1 mg/ml RNase A and incubated at 37°C for 30 min. Propidium iodide was added (final 40 µg/ml), and nuclei were analyzed by flow cytometry.
mRNA expression analysis
RNA isolation from TSCs, cDNA preparation and real-time PCR were performed as described previously (Frank et al., 2001). The primers listed in Table I were used for cDNA amplification. For analysis of cyclin E1 and E2 mRNAs in MEFs, the same primers were also used in conventional PCR reactions (Figure 1B and C).
Table I. Primers used for cDNA amplification.
| Ubiquitin (X51703) | TGGCTATTAATTATTCGGTCTGCAT and GCAAGTGGCTAGAGTGCAGAGTAA |
| pl-1 (M35662) | GCTGGTGTCAAGCCTACTCCTTT and GCAGTTGGTTTGGAGGACACAT |
| plf-1 (NM_031191) | ATAATCATCTCCAAAGCCACAGACA and CCGGACTGCGTTGATCTTTT |
| esx-1 (NM_007957) | TTGCAGAAGCACCGCAAG and AGCCGCCTCCAAGGTTTT |
| flt-1 (NM_010228) | GCTTTATAATAGCAAATGCAACGTACA and CGTTGACGGTGGCTTCG |
| 4311 (NM_009411) | GAAGACTAACAAACTTCACAGTAGCGA and GAGCCTTCCGTCTCCTGGTC |
| eomes (XM_135209) | CCCATCAGATTGTCCCTGGA and CCGGGAAGAAGTTTTGAACG |
| cdx2 (NM_007673) | GCTCCGCAGAACTTTGTCAGT and GTAACCACCGTAGTCCGGGTAC |
| cyclin A (X75483) | AGTTTGATAGATGCTGACCC and TAGGTCTGGTGAAGGTCC |
| cyclin B1 (X64713) | ACTTCCTCCGTAGAGCATC and GCAGAGTTGGTGTCCATTC |
| cyclin E1 (X75888) | TGTTTTTGCAAGACCCAGATGA and GGCTGACTGCTATCCTCGCT |
| cyclin E2 (NM_009830) | GGAACCACAGATGAGGTC and CGTAAGCAAACTCTTGGAG |
| cdk4 (NM_009870) | GCTGGAGGCCTTTGAACATC and CCCGATCAGTTCGGGAAGTAG |
| cdk2 (NM_007673) | TCTGCTCTCACGGGCATTC and AGCTGGAACAGATAGCTCTTGATGA |
| cdc2 (U58633) | ACTCCAGGCTGTATCTCATC and CAAGTCTCTGTGAAGAACTCG |
Acknowledgments
Acknowledgements
We are grateful to Peter Sicinski and Yan Geng for CCNE1-targeted ES cells and for communication throughout this project; and to Sagrario Ortega, Marcos Malumbres and Mariano Barbacid for communicating data on CDK2 knockout mice prior to publication. We thank Freddy Radtke and Michel Aguet for the targeting vector; and Scott Frank, Stefan Taubert, Kristian Helin, Emma Lees, Tyler Jacks, Dan Gorman, Dominique Blanchard, Vivian Barry, Cynthia Ollinger, Cecilia Kim, Annette Bohnert, Kelly Smith, Paul Raihman, Barbara Palmer and Maria A.Ciemerych for discussions, support and/or assistance. This work was funded in part by grants from the National Institutes of Health (CA72006, HD26732 and AG23218) to Z.W. Work in B.A.’s group at the European Institute of Oncology is supported by the Italian Association for Cancer Research (AIRC). DNAX is supported by Schering-Plough Corp.
References
- Ait-Si-Ali S. et al. (1998) Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature, 396, 184–186. [DOI] [PubMed] [Google Scholar]
- Alevizopoulos K., Vlach,J., Hennecke,S. and Amati,B. (1997) Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated Retinoblastoma-family proteins. EMBO J., 16, 5322–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alevizopoulos K., Catarin,B., Vlach,J. and Amati,B. (1998) A novel function of adenovirus E1A is required to overcome growth arrest by the CDK2 inhibitor p27Kip1. EMBO J., 17, 5987–5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow P.W. and Sherman,M.I. (1972) The biochemistry of differentiation of mouse trophoblast: studies on polyploidy. J. Embryol. Exp. Morphol., 27, 447–465. [PubMed] [Google Scholar]
- Beck F., Erler,T., Russell,A. and James,R. (1995) Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev. Dyn., 204, 219–227. [DOI] [PubMed] [Google Scholar]
- Beijersbergen R.L., Carlee,L., Kerkhoven,R.M. and Bernards,R. (1995) Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev., 9, 1340–1353. [DOI] [PubMed] [Google Scholar]
- Bruce J.L., Hurford,R.K. Jr, Classon,M., Koh,J. and Dyson,N. (2000) Requirements for cell cycle arrest by p16INK4a. Mol. Cell, 6, 737–742. [DOI] [PubMed] [Google Scholar]
- Burdon T., Smith,A. and Savatier,P. (2002) Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol., 12, 432–438. [DOI] [PubMed] [Google Scholar]
- Ciemerych M.A., Kenney,A.M., Sicinska,E., Kalaszczynska,I., Bronson,R.T., Rowitch,D.H., Gardner,H. and Sicinski,P. (2002) Development of mice expressing a single D-type cyclin. Genes Dev., 16, 3277–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coverley D., Laman,H. and Laskey,R.A. (2002) Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat. Cell Biol., 4, 523–528. [DOI] [PubMed] [Google Scholar]
- Cross J.C. (2000) Genetic insights into trophoblast differentiation and placental morphogenesis. Semin. Cell Dev. Biol., 11, 105–113. [DOI] [PubMed] [Google Scholar]
- Cross J.C., Werb,Z. and Fisher,S.J. (1994) Implantation and the placenta: key pieces of the development puzzle. Science, 266, 1508–1518. [DOI] [PubMed] [Google Scholar]
- Cross J.C., Flannery,M.L., Blanar,M.A., Steingrimsson,E., Jenkins,N.A., Copeland,N.G., Rutter,W.J. and Werb,Z. (1995) Hxt encodes a basic helix–loop–helix transcription factor that regulates trophoblast cell development. Development, 121, 2513–2523. [DOI] [PubMed] [Google Scholar]
- Dulic V., Lees,E. and Reed,S.I. (1992) Association of human cyclin E with a periodic G1–S phase protein kinase. Science, 257, 1958–1961. [DOI] [PubMed] [Google Scholar]
- Dumont D.J., Fong,G.H., Puri,M.C., Gradwohl,G., Alitalo,K. and Breitman,M.L. (1995) Vascularization of the mouse embryo: a study of flk-1, tek, tie and vascular endothelial growth factor expression during development. Dev. Dyn., 203, 80–92. [DOI] [PubMed] [Google Scholar]
- Duronio R.J. and O’Farrell,P.H. (1995) Developmental control of the G1 to S transition in Drosophila: cyclin E is a limiting downstream target of E2F. Genes Dev., 9, 1456–1468. [DOI] [PubMed] [Google Scholar]
- Ewen M.E. (2000) Where the cell cycle and histones meet. Genes Dev., 14, 2265–2270. [DOI] [PubMed] [Google Scholar]
- Faria T.N., Ogren,L., Talamantes,F., Linzer,D.I. and Soares,M.J. (1991) Localization of placental lactogen-I in trophoblast giant cells of the mouse placenta. Biol. Reprod., 44, 327–331. [DOI] [PubMed] [Google Scholar]
- Follette P.J., Duronio,R.J. and O’Farrell,P.H. (1998) Fluctuations in cyclin E levels are required for multiple rounds of endocycle S phase in Drosophila. Curr. Biol., 8, 235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S.R., Schroeder,M., Fernandez,P., Taubert,S. and Amati,B. (2001) Binding of c-Myc to chromatin mediates mitogen-induced acetylation of histone H4 and gene activation. Genes Dev., 15, 2069–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furstenthal L., Kaiser,B.K., Swanson,C. and Jackson,P.K. (2001a) Cyclin E uses Cdc6 as a chromatin-associated receptor required for DNA replication. J. Cell Biol., 152, 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furstenthal L., Swanson,C., Kaiser,B.K., Eldridge,A.G. and Jackson,P.K. (2001b) Triggering ubiquitination of a CDK inhibitor at origins of DNA replication. Nat. Cell Biol., 3, 715–722. [DOI] [PubMed] [Google Scholar]
- Geng Y. et al. (2001) Expression of cyclins E1 and E2 during mouse development and in neoplasia. Proc. Natl Acad. Sci. USA, 98, 13138–13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y. et al. (2003) Cyclin E ablation in the mouse. Cell, 114, 431–443. [DOI] [PubMed] [Google Scholar]
- Guan K.L., Jenkins,C.W., Li,Y., Nichols,M.A., Wu,X., O’Keefe,C.L., Matera,A.G. and Xiong,Y. (1994) Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev., 8, 2939–2952. [DOI] [PubMed] [Google Scholar]
- Gudas J.M., Payton,M., Thukral,S., Chen,E., Bass,M., Robinson,M.O. and Coats,S. (1999) Cyclin E2, a novel G1 cyclin that binds Cdk2 and is aberrantly expressed in human cancers. Mol. Cell. Biol., 19, 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour J., Luo,R., Dei Santi,A., Postigo,A. and Dean,D. (1999) Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell, 98, 859–869. [DOI] [PubMed] [Google Scholar]
- Hoffmann I., Draetta,G. and Karsenti,E. (1994) Activation of the phosphatase activity of human cdc25A by a cdk2–cyclin E dependent phosphorylation at the G1/S transition. EMBO J., 13, 4302–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P.K., Chevalier,S., Philippe,M. and Kirschner,M.W. (1995) Early events in DNA replication require cyclin E and are blocked by p21CIP1. J. Cell Biol., 130, 755–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B.L., Wolfe,K.G. and Roberts,J.M. (1998) Identification of a substrate-targeting domain in cyclin E necessary for phosphorylation of the retinoblastoma protein. Proc. Natl Acad. Sci. USA, 95, 2535–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich J.A., Sauer,K., Jones,L., Richardson,H., Saint,R. and Lehner,C.F. (1994) Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell, 77, 107–120. [DOI] [PubMed] [Google Scholar]
- Koff A. et al. (1992) Formation and activation of a cyclin E–cdk2 complex during the G1 phase of the human cell cycle. Science, 257, 1689–1694. [DOI] [PubMed] [Google Scholar]
- Kohn M.J., Bronson,R.T., Harlow,E., Dyson,N.J. and Yamasaki,L. (2003) Dp1 is required for extra-embryonic development. Development, 130, 1295–1305. [DOI] [PubMed] [Google Scholar]
- Kraut N., Snider,L., Chen,C.M., Tapscott,S.J. and Groudine,M. (1998) Requirement of the mouse I-mfa gene for placental development and skeletal patterning. EMBO J., 17, 6276–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krude T., Jackman,M., Pines,J. and Laskey,R.A. (1997) Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell, 88, 109–119. [DOI] [PubMed] [Google Scholar]
- Lauper N., Beck,A.R.P., Cariou,S., Richman,L., Hoffmann,K., Reith,W., Slingerland,J.M. and Amati,B. (1998) Cyclin E2: a novel CDK2 partner in the late G1 and S phases of the mammalian cell cycle. Oncogene, 17, 2637–2643. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Talamantes,F., Wilder,E., Linzer,D.I. and Nathans,D. (1988) Trophoblastic giant cells of the mouse placenta as the site of proliferin synthesis. Endocrinology, 122, 1761–1768. [DOI] [PubMed] [Google Scholar]
- Lescisin K.R., Varmuza,S. and Rossant,J. (1988) Isolation and characterization of a novel trophoblast-specific cDNA in the mouse. Genes Dev., 2, 1639–1646. [DOI] [PubMed] [Google Scholar]
- Lukas J., Parry,D., Aagaard,L., Mann,D.J., Bartkova,J., Strauss,M., Peters,G. and Bartek,J. (1995) Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature, 375, 503–506. [DOI] [PubMed] [Google Scholar]
- Lukas J., Herzinger,T., Hansen,K., Moroni,M., Resnitzky,D., Helin,K., Reed,S. and Bartek,J. (1997) Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev., 11, 1479–1492. [DOI] [PubMed] [Google Scholar]
- Lundberg A.S. and Weinberg,R.A. (1998) Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin–Cdk complexes. Mol. Cell. Biol., 18, 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Sladek,R., Bader,J.A., Matthyssen,A., Rossant,J. and Giguere,V. (1997) Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-β. Nature, 388, 778–782. [DOI] [PubMed] [Google Scholar]
- Ma T. et al. (2000) Cell cycle-regulated phosphorylation of p220(NPAT) by cyclin E/Cdk2 in Cajal bodies promotes histone gene transcription. Genes Dev., 14, 2298–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAuley A., Cross,J.C. and Werb,Z. (1998) Reprogramming the cell cycle for endoreduplication in rodent trophoblast cells. Mol. Biol. Cell, 9, 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek N.P., Sundberg,H., McGrew,S., Nakayama,K., Kyriakides,T.R., Roberts,J.M. and Kyriakidis,T.R. (2001) A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature, 413, 323–327. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Ewen,M.E., Strom,D.K., Kato,J.Y., Hanks,S.K., Roussel,M.F. and Sherr,C.J. (1992) Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell, 71, 323–334. [DOI] [PubMed] [Google Scholar]
- Medema R.H., Herrera,R.E., Lam,F. and Weinberg,R.A. (1995) Growth suppression by p16INK4 requires functional retinoblastoma protein. Proc. Natl Acad. Sci. USA, 92, 6289–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnoli A., Fiore,F., Eytan,E., Carrano,A.C., Draetta,G.F., Hershko,A. and Pagano,M. (1999) Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev., 13, 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.D., Kirk,J.A. and Hunt,T. (2003) Unmasking the S-phase-promoting potential of cyclin B1. Science, 300, 987–990. [DOI] [PubMed] [Google Scholar]
- Murphy M., Stinnakre,M.G., Senamaud-Beaufort,C., Winston,N.J., Sweeney,C., Kubelka,M., Carrington,M., Brechot,C. and Sobczak-Thepot,J. (1997) Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nature Genet., 15, 83–86. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Theodoras,A.M., Schumacher,J., Roberts,J.M. and Pagano,M. (1995) Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol. Cell. Biol., 15, 2612–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda M. et al. (2000) Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell, 103, 127–140. [DOI] [PubMed] [Google Scholar]
- Ortega S., Prieto,I., Odajima,J., Martín,A., Dubus,P., Sotillo,R., Barbero,J.L., Malumbres,M. and Barbacid,M. (2003) Cyclin dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet., in press (doi: 10.1038/ng1232). [DOI] [PubMed] [Google Scholar]
- Polyak K., Kato,J.Y., Solomon,M.J., Sherr,C.J., Massague,J., Roberts,J.M. and Koff,A. (1994) p27Kip1, a cyclin–Cdk inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes Dev., 8, 9–22. [DOI] [PubMed] [Google Scholar]
- Radtke F., Wilson,A., Stark,G., Bauer,M., van Meerwijk,J., MacDonald,H.R. and Aguet,M. (1999) Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity, 10, 547–558. [DOI] [PubMed] [Google Scholar]
- Russ A.P. et al. (2000) Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature, 404, 95–99. [DOI] [PubMed] [Google Scholar]
- Russo A.A., Jeffrey,P.D., Patten,A.K., Massagué,J. and Pavletich,N.P. (1996) Crystal structure of the p27Kip1 cyclin-dependent kinase inhibitor bound to the cyclin A–Cdk2 complex. Nature, 382, 325–331. [DOI] [PubMed] [Google Scholar]
- Sauer K., Knoblich,J.A., Richardson,H. and Lehner,C.F. (1995) Distinct modes of cyclin E/cdc2c kinase regulation and S-phase control in mitotic and endoreduplication cycles of Drosophila embryogenesis. Genes Dev., 9, 1327–1339. [DOI] [PubMed] [Google Scholar]
- Schorpp-Kistner M., Wang,Z.Q., Angel,P. and Wagner,E.F. (1999) JunB is essential for mammalian placentation. EMBO J., 18, 934–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghezzi W., Chua,K., Shanahan,F., Gozani,O., Reed,R. and Lees,E. (1998) Cyclin E associates with components of the pre-mRNA splicing machinery in mammalian cells. Mol. Cell. Biol., 18, 4526–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan F., Seghezzi,W., Parry,D., Mahony,D. and Lees,E. (1999) Cyclin E associates with BAF155 and BRG1, components of the mammalian SWI–SNF complex and alters the ability of BRG1 to induce growth arrest. Mol. Cell. Biol., 19, 1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaff R.J., Groudine,M., Gordon,M., Roberts,J.M. and Clurman,B.E. (1997) Cyclin E–CDK2 is a regulator of p27Kip1. Genes Dev., 11, 1464–1478. [DOI] [PubMed] [Google Scholar]
- Shen-Li H., O’Hagan,R.C., Hou,H. Jr, Horner,J.W. 2nd, Lee,H.W. and DePinho,R.A. (2000) Essential role for Max in early embryonic growth and development. Genes Dev., 14, 17–22. [PMC free article] [PubMed] [Google Scholar]
- Sherr C.J. and Roberts,J.M. (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev., 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Stead E. et al. (2002) Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene, 21, 8320–8333. [DOI] [PubMed] [Google Scholar]
- Su T.T. and O’Farrell,P.H. (1997) Chromosome association of minichromosome maintenance proteins in Drosophila mitotic cycles. J. Cell Biol., 139, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T.T. and O’Farrell,P.H. (1998) Chromosome association of minichromosome maintenance proteins in Drosophila endoreplication cycles. J. Cell Biol., 140, 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Kunath,T., Hadjantonakis,A.K., Nagy,A. and Rossant,J. (1998) Promotion of trophoblast stem cell proliferation by FGF4. Science, 282, 2072–2075. [DOI] [PubMed] [Google Scholar]
- Tetsu O. and McCormick,F. (2003) Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell, 3, 233–245. [DOI] [PubMed] [Google Scholar]
- Tremblay K.D., Dunn,N.R. and Robertson,E.J. (2001) Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development, 128, 3609–3621. [DOI] [PubMed] [Google Scholar]
- Varmuza S., Prideaux,V., Kothary,R. and Rossant,J. (1988) Polytene chromosomes in mouse trophoblast giant cells. Development, 102, 127–134. [DOI] [PubMed] [Google Scholar]
- Vlach J., Hennecke,S., Alevizopoulos,K., Conti,D. and Amati,B. (1996) Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J., 15, 6595–6604. [PMC free article] [PubMed] [Google Scholar]
- Vlach J., Hennecke,S. and Amati,B. (1997) Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J., 16, 5334–5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. et al. (2003) Extra-embryonic function of Rb is essential for embryonic development and viability. Nature, 421, 942–947. [DOI] [PubMed] [Google Scholar]
- Xiao Z.X., Ginsberg,D., Ewen,M. and Livingston,D.M. (1996) Regulation of the retinoblastoma protein-related protein p107 by G1 cyclin-associated kinases. Proc. Natl Acad. Sci. USA, 93, 4633–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.T., Stein,S.M., Ding,J., Shen,M.M. and Abate-Shen,C. (2000) A novel PF/PN motif inhibits nuclear localization and DNA binding activity of the ESX1 homeoprotein. Mol. Cell. Biol., 20, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala M., Liu,J. and Xiong,Y. (1998) Cyclin E2, a novel human G1 cyclin and activating partner of CDK2 and CDK3, is induced by viral oncoproteins. Oncogene, 17, 2787–2798. [DOI] [PubMed] [Google Scholar]
- Zhang P., Wong,C., DePinho,R.A., Harper,J.W. and Elledge,S.J. (1998) Cooperation between the Cdk inhibitors p27KIP1 and p57KIP2 in the control of tissue growth and development. Genes Dev., 12, 3162–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. et al. (2000) NPAT links cyclin E–Cdk2 to the regulation of replication-dependent histone gene transcription. Genes Dev., 14, 2283–2297. [PMC free article] [PubMed] [Google Scholar]
- Zybina E.V. and Zybina,T.G. (1996) Polytene chromosomes in mammalian cells. Int. Rev. Cytol., 165, 53–119. [DOI] [PubMed] [Google Scholar]