Abstract
Intracellular signaling pathways, which regulate the interactions of integrins with their ligands, affect a wide variety of biological functions. Here we provide evidence of how cytohesin-1, an integrin-binding protein and guanine-nucleotide exchange factor (GEF) for ARF GTPases, regulates cell adhesion. Mutational analyses of the β-2 cytoplasmic domain revealed that the adhesive function of LFA-1 depends on its interaction with cytohesin-1, unless the integrin is activated by exogenous divalent cations. Secondly, cytohesin-1 induces expression of an extracellular activation epitope of LFA-1, and the exchange factor function is not essential for this activity. In contrast, LFA-1-mediated cell adhesion and spreading on intercellular cell adhesion molecule 1 is strongly inhibited by a cytohesin-1 mutant, which fails to catalyze ARF GDP–GTP exchange in vitro. Thus, cytohesin-1 is involved in the activation of LFA-1, most probably through direct interaction with the integrin, and induces cell spreading by its ARF-GEF activity. We therefore propose that both direct regulation of the integrin and concomitant changes in the membrane topology of adherent T cells are modulated by dissectable functions of cytohesin-1.
Keywords: ARF GTPase/cell adhesion/cytohesin-1/β-2 integrin/LFA-1
Introduction
Integrins are a family of α/β heterodimeric adhesion receptors with diverse biological roles in leukocyte functions, development, wound healing (Hynes, 1992) and neurological memory (Grotewiel et al., 1998). There is a range of abilities amongst integrins to bind ligand, which may either be a cell surface counter-receptor or an extracellular matrix component (Hynes, 1992). A number of studies have shown that the binding of integrins to their ligands is regulated by diverse and complex intracellular signal transduction pathways, which in turn are triggered by cell surface receptors controlling the biological function of integrins in various tissues (Hynes, 1992; Sastry and Horwitz, 1993; Diamond and Springer, 1994). In T cells, activating proteins include chemokine receptors, cytokine receptors, the T-cell antigen receptor (TCR) and co-stimulatory molecules such as CD2, CD7 or CD28 (Dustin and Springer, 1989; van Kooyk et al., 1989; Shimizu et al., 1995; Carr et al., 1996; Nielsen et al., 1996; Zell et al., 1996; Chan et al., 1997). Mechanistic models attempting to explain the observed phenomena have been proposed. These include arguments for direct affinity regulation of integrin molecules through conformational changes. On the other hand, there is evidence for avidity switch scenarios, including enhancement of lateral mobility of integrins, and/or cytoskeletal remodeling events, allowing receptor clustering and effective cell spreading (Lub et al., 1995; Kolanus and Seed, 1997; Hemler, 1998; Hughes and Pfaff, 1998). Some researchers favor proteolytic events as a regulatory mechanism (Fox et al., 1993; Du et al., 1995; Stewart et al., 1998). However, the integrin-proximal molecular events in ‘inside-out’ signal transduction are not well understood.
Recently, a number of cytoplasmic molecules have been identified that interact with the intracellular tails of integrin receptors, and it is believed that the detailed analysis of their biological roles may help to unravel the mechanisms that ultimately lead to integrin avidity or affinity modulation (activation) (Hemler, 1998; Huang and Wu, 1999). One of these molecules is cytohesin-1, a 47 kDa cytoplasmic protein that is expressed predominantly in hematopoietic cells (Kolanus et al., 1996). Cytohesin-1 comprises an N-terminal coiled-coil domain, which may aid in oligomerization, a central Sec7 domain and a C-terminal module including the pleckstrin homology (PH) domain and a polybasic region. Cytohesin-1 specifically binds to the intracellular domain of the β-2 integrin chain and has been implicated in the functional regulation of the leukocyte integrin LFA-1 (CD11a/CD18, αLβ2). Overexpression of cytohesin-1 resulted in the enhanced binding of Jurkat cells to the β-2 integrin ligand intercellular cell adhesion molecule 1 (ICAM-1), whereas the expression of the C-terminal module abrogated stimulus-dependent activation of LFA-1 in a dominant-negative fashion (Kolanus et al., 1996). Subsequent studies showed that the C-terminal portions of cytohesin-1, the PH domain and the polybasic region cooperated in high affinity binding of the molecule to phosphatidylinositol-(3,4,5)-trisphosphate, a cellular phosphorylation product of phosphatidylinositol 3-kinase (PI3-kinase; Nagel et al., 1998a,b; Venkateswarlu et al., 1999). It was shown, furthermore, that LFA-1 is regulated directly by PI3-kinase in T cells, through intermediate membrane recruitment of cytohesin-1 (Nagel et al., 1998b). A similar mechanism of integrin activation, i.e. both PI3-kinase and cytohesin-1 dependent, subsequently was proposed for lipopolysaccharide (LPS)-mediated activation of LFA-1 in monocytes through CD14 (Hmama et al., 1999). The precise function of cytohesin-1 in this context is as yet unclear. The molecule belongs to a class of cytoplasmic exchange factors for small GTPases called ADP ribosylation factors or ARFs (Meacci et al., 1997). ARFs have been implicated predominantly in the regulation of intracellular vesicle transport, affecting both Golgi and endosomal functions (reviewed by Moss and Vaughan, 1998). However, recent evidence from several laboratories points to a possible role for ARF G-proteins in the remodeling of the plasma membrane actin cytoskeleton. Norman et al. (1998) have shown that the deposition of the actin-linker protein paxilin at focal adhesions of fibroblasts was inhibited by a dominant-negative mutant of ARF1. Furthermore, ARF6 was implicated in membrane ruffling and actin cytoskeletal reorganization (D’Souza et al., 1997; Frank et al., 1998; Franco et al., 1999). In this study, we show that cytohesin-1 acts as a GDP–GTP exchange factor in the regulation of LFA-1, because overexpression of a mutant that abrogates GEF activity for ARF proteins in vitro results in dominant-negative inhibition of Jurkat and peripheral blood lymphocyte (PBL) binding to ICAM-1. Furthermore, we identified a β-2 cytoplasmic domain mutant of LFA-1 that fails to interact with cytohesin-1 in vitro.
Expression of this mutant in two different T-cell lines that lack endogenous β-2 chains results in its failure to become activated by either TCR stimulation or phorbol ester treatment. However, the mutant is expressed normally and responds to divalent cation stimulation, which is thought to act exclusively at the level of the extracellular domains, thus bypassing intracellular signaling events.
With respect to LFA-1 activation, cytohesin-1 functions at two discernable levels, through its ARF-GEF activity and via direct interaction with the integrin.
Results
Endogenous LFA-1 and cytohesin-1 co-localize in human lymphoblastoid B cells
A rat monoclonal antibody (mAb7H2) was generated that recognizes human cytohesin-1, but not ARNO (cytohesin-2) (Figure 1A). With the help of Western blot analyses, we showed that cytohesin-1 is expressed abundantly in hematopoietic cell types (Figure 1B), but we also observed detectable expression of the molecule in non-hematopoietic lines, such as HeLa cells (Figure 1B) or K562 cells. Notably, high-level expression of cytohesin-1 correlates very well with that of LFA-1 (data not shown).
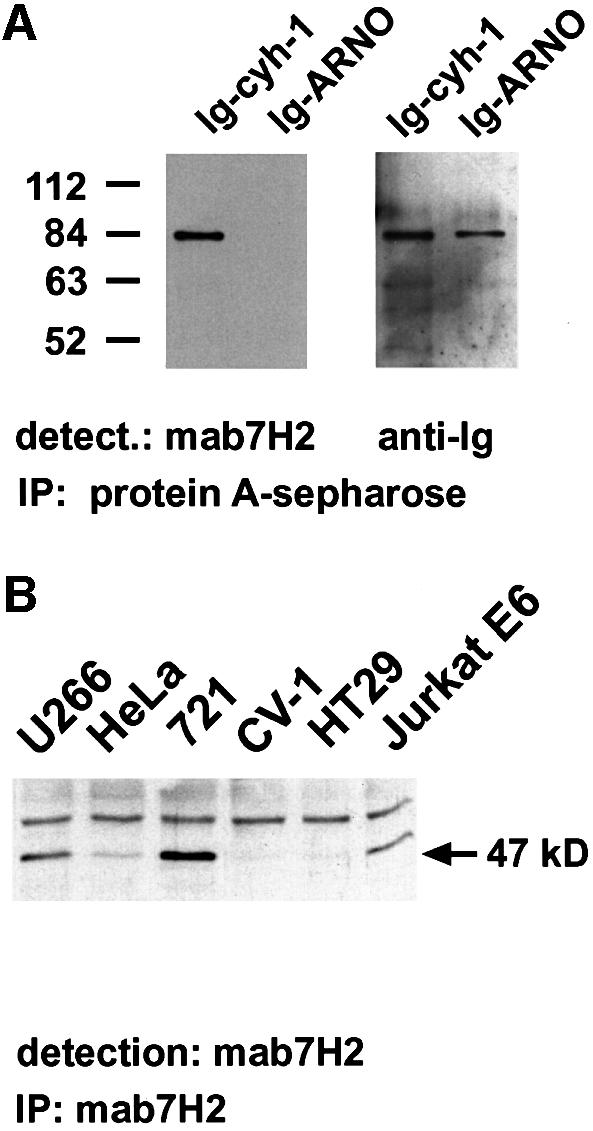
Fig. 1. Specificity of anti-cytohesin-1 monoclonal antibody mAb7H2. (A) To demonstrate the specificity of mAb7H2, Ig fusions of human cytohesin-1 and human ARNO (cytohesin-2) were precipitated on protein A–Sepharose. In the subsequent immunoblot analysis, cytohesin-1, but not ARNO, is detected by mAb7H2. (B) Cytohesin-1 was precipitated from various human cell lines by mAb7H2, and protein expression was monitored by immunoblot analysis: the protein is present in U266 cells (lymphoblastoid myeloma line), 721-LCL cells (Burkitt lymphoma), Jurkat E6 cells (T-cell leukemia) and at reduced levels in HeLa cells (cervical carcinoma line). Hardly any, or no cytohesin-1 expression is found in HT29 cells (adenocarcinoma line) or in CV-1 cells (African green monkey kidney line). The upper band corresponds to the heavy chain of the monoclonal antibody.
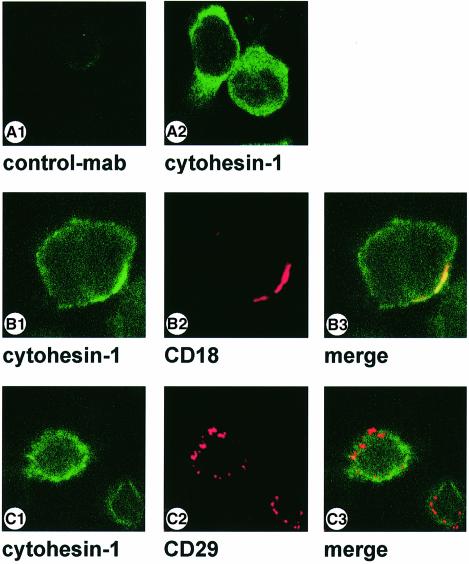
We subsequently used mAb7H2 to analyze the cellular co-localization of LFA-1 and cytohesin-1. Several lymphoid cell lines, as well as PBLs and cytotoxic T cells, were tested and yielded similar results (Figure 2, and data not shown). An example is shown in Figure 2: LFA-1, which has been clustered by antibodies, and cytohesin-1 co-localize well at the plasma membrane of lymphoblastoid 721-LCL cells (Figure 2B); this observation suggests a tight cellular association of the molecules. Specificity controls for these data are given in Figure 2A and C: an isotype-matched control antibody (CAD9) does not stain permeabilized 721-LCL cells (Figure 2A), and antibody-mediated clustering of the endogenous β1-integrin receptor on the same cell type does not result in co-localization of cytohesin-1 (Figure 2C).
Fig. 2. Co-localization of cytohesin-1 with CD18 in the human lymphoblastoid cell line 721-LCL. 721-LCL cells were incubated with either anti-human CD18 mouse monoclonal antibody KIM185 (B) or anti-human CD29-antibody 4B7R (C) at 1 µg/ml for 10 min, followed by cross-linking with secondary goat anti-mouse Texas red-conjugated F(ab′)2 fragment at 37°C. Cells were then plated on poly-l-lysine-coated slides as described in Materials and methods. For staining of endogenous cytohesin-1, cells subsequently were permeabilized and incubated with rat hybridoma supernatants containing the anti-cytohesin mAb. Detection of the rat immunoglobulin was performed with an FITC-conjugated anti-rat secondary reagent. Anti-cytohesin-1 staining is specific, because the isotype-matched rat control antibody CAD9 did not yield detectable fluorescence (A). Cross-species reactivity of the secondary reagents was not detectable (not shown).
An LFA-1 mutant that fails to interact with cytohesin-1 is defective in cell adhesion
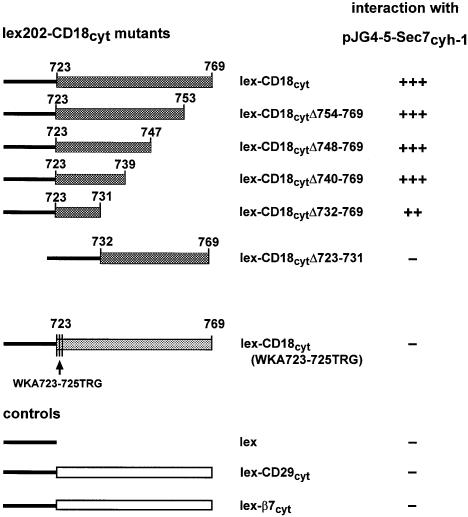
We then investigated in more detail whether the interaction of CD18 with cytohesin-1 was required for LFA-1 adhesion to ICAM-1. To this end, we first mapped the region within the CD18 cytoplasmic domain that was responsible for the interaction with cytohesin-1. Deletion analysis performed with the help of the yeast two-hybrid system (Fields and Song, 1989; Gyuris et al., 1993) showed that the nine membrane-proximal amino acids of the CD18 cytoplasmic tail retained the ability to interact with the Sec7 domain of cytohesin-1 or with full-length cytohesin-1 (Figure 3; data not shown). Several individual point mutants of this region subsequently were generated by site-directed mutagenesis and tested for interaction with the Sec7 domain of cytohesin-1 in yeast, but none of them was capable of abrogating the interaction completely (not shown). However, a triple mutant CD18(WKA723–725TRG) was discovered by this method, which was characterized by a complete loss of interaction with the cytohesin-1 Sec7 domain in yeast. Amino acids WKA723–25 correspond to the membrane-proximal segment of the CD18 cytoplasmic domain (Figure 3).
Fig. 3. Mapping of the cytohesin-1-binding site within the cytoplasmic domain of CD18, using the yeast two-hybrid system. Deletion mutants of the CD18 cytoplasmic domain and control constructs were expressed as fusion proteins with the LexA DNA-binding domain (bait) and co-expressed with fusion proteins comprising the Sec7 domain of cytohesin-1 in the context of the B42 transactivation domain (prey). Deletion analysis shows that nine membrane-proximal amino acids of the CD18 cytoplasmic tail, including amino acids 723–731, retained the ability to interact with cytohesin-1 (++) compared with the strong interaction with the full-length cytoplasmic tail of CD18 (+++). In contrast, a triple mutation (WKA723–725TRG) of the N-terminal three amino acids of the CD18 cytoplasmic domain completely abrogates the interaction with cytohesin-1. No interaction (–) was detected with the LexA protein alone, or with the CD29 and β-7 integrin cytoplasmic domain fusion proteins. Interaction studies were performed in the yeast strain EGY48/JK103 and monitored by the generation of blue dye as a result of the interaction-dependent lacZ gene activity in yeast cells grown on medium containing X-gal and galactose. The strength of interaction was scored as (–) negative, positive (++) and strongly positive (+++).
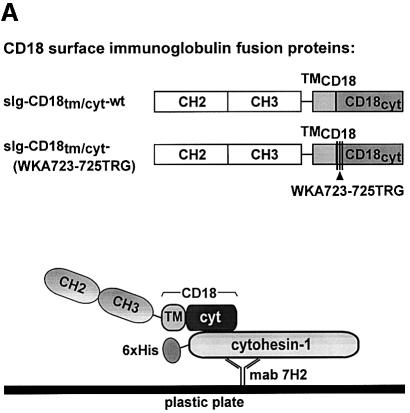
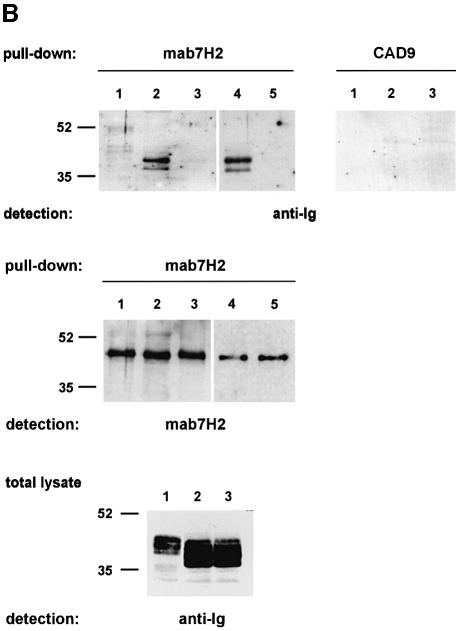
The yeast data were confirmed by a pull-down/immunoprecipitation assay (Figure 4). Specifically, single chain immunoglobulin chimeras, bearing both transmembrane and cytoplasmic portions of wild-type CD18 or of CD18(WKA723–725TRG) (Figure 4A), were expressed in COS cells, and the cells subsequently were lysed. From the lysates, the recombinant CD18 chimeras were bound to His6-cytohesin-1 from Escherichia coli (Kolanus et al., 1996) and immobilized by the anti-cytohesin-1 antibody mAb7H2 (Figure 4A). We found that the chimera bearing the wild-type CD18 sequence was precipitated effectively from total cell lysates by cytohesin-1 in vitro (Figure 4B, upper left panel, lane 2) whereas a control protein (upper left panel, lane 1) or the CD18(WKA723–25TRG) (Figure 4B, upper left panel, lane 3) mutant were not enriched by cytohesin-1 above background levels. An isotype-matched control antibody (CAD9), which fails to immobilize cytohesin-1, consequently does not precipitate any recombinant chimeric molecules from the cell lysates (Figure 4B, upper right panel). Interestingly, a cytohesin-1 mutant in which the ARF-GEF function of the molecule is disrupted [cytohesin-1(E157K), see the detailed analysis of the mutant below] and wild-type cytohesin-1 showed similar interactions with the CD18 fusion protein in these experiments (Figure 4B, upper left panel, lanes 4 and 5).
Fig. 4. The sIg–CD18cyt wild-type fusion protein, but not the sIg–CD18tm/cyt-WKA723–725TRG mutant, binds to cytohesin-1 in vitro. Biochemical evaluation of an interaction between CD18 and cytohesin-1 was performed by an in vitro pull-down assay. The experiment employed single chain fusion proteins, expressed in COS cells, and purified His6-cytohesin-1 from E.coli. (A) Schematic outline of the CD18 surface immunoglobulin fusion proteins used in this study (upper panel) and schematic of the pull-down experiment (lower panel). Surface immunoglobulin (sIg) chimeras comprised the CH2 and CH3 domains from human immunoglobulin G1, fused to the transmembrane and cytoplasmic domains of CD18. Surface expression was directed by the leader sequence of CD5 (the sketched domain sizes are not proportional to the actual amino acid sequences). (B) For pull-down of CD18 fusion proteins, 96-well microtiter plates were coated with the monoclonal antibody mAb7H2 to immobilize polyhistidine-tagged cytohesin-1 (lanes 1, 2 and 3) or cytohesin-1E157K (lanes 4 and 5), respectively. Subsequently, wells were incubated with lysates from COS cells expressing (lane 1) a surface Ig-control chimera bearing the transmembrane domain of CD7, (lanes 2 and 4) wild-type sIg–CD18tm/cyt and (lanes 3 and 5) sIg–CD18tm/cyt-WKA723–725TRG. Precipitated sIg proteins were collected and monitored by immunoblot analysis. Specific interaction of cytohesin-1 and cytohesin-1E157K with CD18 was confirmed by detection of the precipitated sIg–CD18tm/cyt-wild-type fusion protein (lanes 2 and 4, upper left panel) with an anti-human IgG antibody. Neither sIg control protein (lane 1) nor the triple mutant of CD18, sIg–CD18tm/cyt-WKA723–725TRG (lanes 3 and 5) revealed significant binding to recombinant cytohesin-1. A control was also performed with the mAb7H2 isotype-specific antibody CAD9. The amount of antibody-captured His6-cytohesin-1 contained within each sample (middle panel) and expression of the sIg fusion proteins (lower panel) was verified by immunoblotting with mAb7H2 and anti-human IgG antibody, respectively.
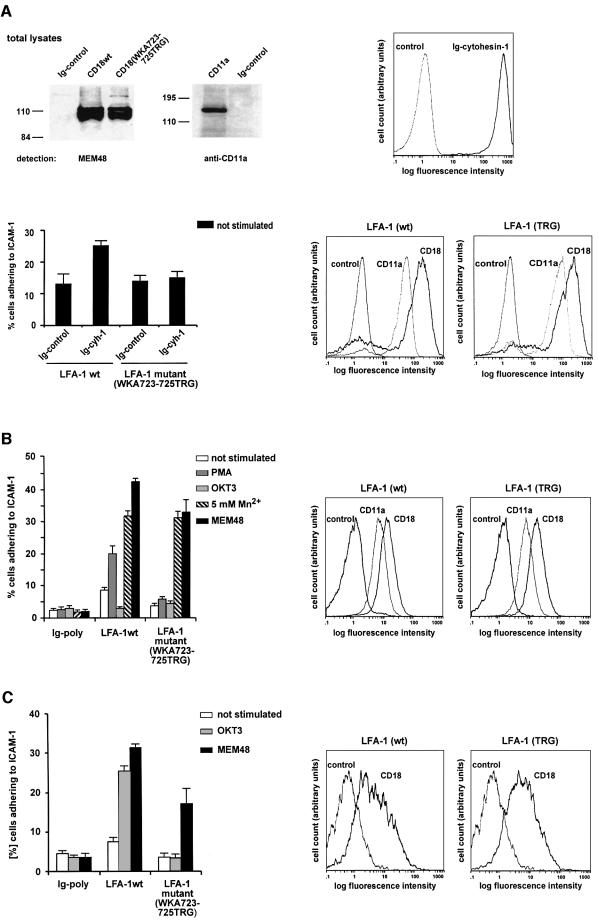
In order to test whether cell adhesion to ICAM-1 was affected by the introduction of these mutations, the WKA723–725TRG mutation was transferred genetically into the context of an intact β-2 chain. Recombinant vaccinia viruses subsequently were generated producing wild-type CD18, CD18(WKA723–725TRG) or the wild-type α chain (CD11a). Expression in HeLa cells showed that the electrophoretic mobility pattern of the individual polypeptide chains corresponded to the expected molecular masses (Figure 5A, upper left panel). Detection of both wild-type and mutant β chains at the cell surface required the presence of an intact α chain (data not shown); the β chains thus behaved similarly with respect to both expression levels and α chain-dependent transport to the cell surface.
Fig. 5. The adhesive capacity of the CD18(WKA723–725TRG) mutant is activated neither by overexpression of cytohesin-1 nor by stimulation through TCR- or phorbol ester-mediated signal transduction events. Assays were performed with HeLa cells expressing vaccinia virus-derived recombinant LFA-1 molecules, and with HeLa cells stably expressing LFA-1 (A). Additional adhesions were performed with hematopoietic, β-2 chain-deficient cell lines (B and C). (A) Upper left panel: Western blot analysis of recombinant vaccinia virus-derived CD18 and CD11a proteins. Wild-type α chain CD11a, wild-type CD18 or the mutant CD18(WKA723–725TRG) were expressed with recombinant vaccinia virus. Expression of CD18 is detected as a cluster of bands migrating between 100 and 120 kDa with the monoclonal antibody MEM48 under non-reducing conditions. CD11a was detected with the anti-CD11a antibody MHM24 (Dako) at 180 kDa. As a control, cells have been infected with a cytoplasmic Ig control protein. Upper right panel: an intracellular Ig–cytohesin-1 fusion protein is expressed in HeLa cells with the help of recombinant vaccinia viruses. The fusion protein was detected by flow cytometric analysis following fixation and permeabilization of the cells. Lower right panel: HeLa cells stably expressing either wild-type LFA-1 or the TRG triple mutant were established, and cell surface expression was measured by flow cytometry using the antibodies MEM48 (CD18) and MHM24 (CD11a). Lower left panel: adhesion assays with HeLa cells stably expressing wild-type and mutant versions of LFA-1 were performed. Overexpression of Ig–cytohesin-1, but not of the control construct, increases adhesion of LFA-1 wild-type-positive cells, but not of LFA-1 TRG mutant-positive cells. (B) Right panel: expression of either wild-type or mutant β-2 chain rescues cell surface expression of LFA-1 in β-2-deficient SKβ2.7 cells. Left panel: adhesion assay. Wild-type LFA-1, but not the CD18(WKA723–725TRG) mutant, responds to PMA stimulation in SKβ2.7 cells (gray bars). However, both receptors respond well to Mn2+ or to the activating anti-LFA-1 antibody MEM-48 (hatched or solid bars, respectively). (C) Right panel: expression of either wild-type or mutant β-2 chain rescues cell surface expression of LFA-1 in β-2-deficient LAD19 cells. Left panel: adhesion assay. Wild-type LFA-1, but not the CD18(WKA723–725TRG) mutant, responds to monclonal antibody OKT3 (anti-TCR)-mediated stimulation of adhesion to ICAM-1 in LAD19 cells (gray bars). However, both receptors respond to activating antibody MEM-48 (solid bars).
Our data predicted that the LFA-1-CD18(WKA723–725TRG) mutant should fail to mediate enhanced adhesion following cytohesin-1 overexpression. To test this, we generated stable HeLa cell lines expressing wild-type and mutant recombinant LFA-1 molecules. This was done because the transient system did not yield reproducible, simultaneous overexpression of both integrin receptor chains and cytohesin-1 (not shown). Therefore, stable expression constructs of wild-type or mutant receptor chains were generated using a bicistronic expression cassette, and the puromycin acetyltransferase gene was employed as a selectable marker. Double transfections and subsequent selections yielded stably expressed LFA-1 molecules at very high cell surface densities (Figure 5A, lower right panel), as detected by flow cytometric analysis. Employing these cells in our adhesion assays (Figure 5A, lower left panel), we found that basal adhesion levels of both stable cell lines were substantially higher when compared with the transiently expressed receptors. This finding was correlated strongly to the very high level of expression in the stable expression system (data not shown).
These stable cell lines were infected subsequently with recombinant vaccinia viruses expressing a chimeric derivative of cytohesin-1 (Ig–cytohesin-1; Kolanus et al., 1996). The recombinant fusion protein was expressed at high levels (Figure 5A, upper right panel). The infected cells were then subjected to adhesion analysis (Figure 5A, lower left panel). We observed that stably expressed wild-type LFA-1, but not the mutant molecule, responded to specific overexpression of Ig–cytohesin-1 by a 2-fold enhanced adhesion to ICAM-1.
Ectopically expressed LFA-1 does not respond well to intracellular stimuli, such as phorbol esters (Lub et al., 1997b; our own data with LFA-1-HeLa cell lines, not shown). To test whether the CD18(WKA723–725TRG) mutant was capable of responding to intracellular signals, we introduced both the wild-type and mutant β chains into the β-2 chain-deficient T-cell leukemia line SK-β2.7 (Weber et al., 1997). Figure 5B (right panel) documents that vaccinia virus-derived expression of wild-type CD18 and that of CD18(WKA723–725TRG) rescued cell surface expression of LFA-1 in these cells. Subsequently, adhesion assays were performed and the results are shown in Figure 5B (left panel). Infection of SK-β2.7 with control construct did not yield significant adhesion to ICAM-1, since LFA-1 was not detectable at the cell surface (Figure 5B, and data not shown). Introduction of wild-type CD18 resulted in a significant enhancement of basal adhesion, which was stimulated a further 2.5-fold by pre-incubating the cells with the phorbol ester PMA. These results are in full agreement with the previously published initial characterization of SK-β2.7 cells using stable expression of the wild-type β-2 chain (Weber et al., 1997). Anti-TCR antibody treatment of these cells did not result in enhanced adhesion (Figure 5B), which was explained by the finding that the cells did not express significant levels of the TCR at the cell surface (not shown). Importantly, the CD18(WKA723–725TRG) mutant exhibited a lower level of basal adhesion, which could not be stimulated further by PMA (Figure 5B). This functional deficiency apparently was not due to structural defects of the integrin mutant, because both wild-type and mutant proteins were activated to equal levels by exogenous Mn2+ or Mg2+ (Figure 5B, and data not shown), an activation stimulus that presumably acts independently of intracellular functions (Dransfield et al., 1992). Furthermore, an anti-LFA-1 antibody (MEM-48), which is known to activate adhesion to ICAM-1, rescues the function of both wild-type and mutant receptors.
We then employed β-2-deficient LAD19 T-cell clones (Vennegoor et al., 1992) to test whether the CD18(WKA723–725TRG) mutant was capable of responding to anti-TCR stimuli, because TCR expression was detectable on the surfaces of these cells. Figure 5C (left panel) shows that although wild-type CD18 responded well to TCR-mediated signals, CD18(WKA723–725TRG) failed to mediate significant adhesion to ICAM-1 when tested under identical conditions. Taken together, these data demonstrate in various ways that cytohesin-1 is an important determinant of LFA-1-mediated cellular adhesion to ICAM-1.
Both cytohesin-1 and a guanine nucleotide exchange function-defective mutant of cytohesin-1 induce an extracellular activation epitope of LFA-1
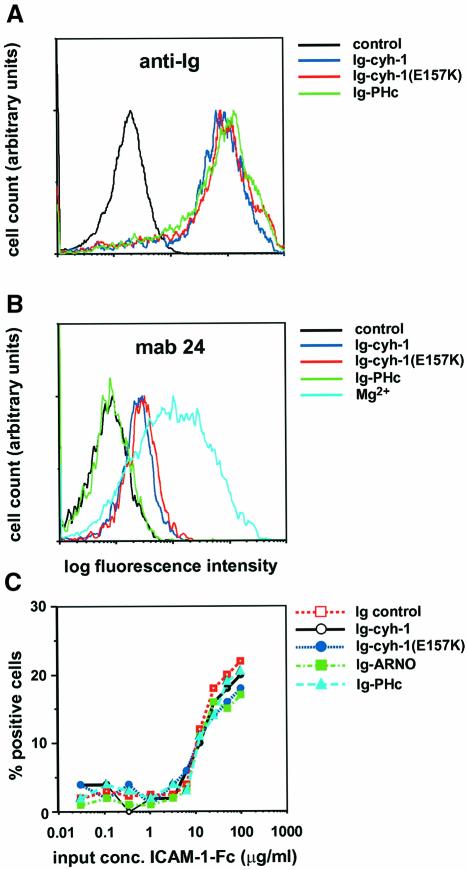
We had shown earlier that LFA-1-mediated cell adhesion could be modulated by overexpression of cytohesin-1, or of subdomains thereof. It was as yet unclear whether this directly affected LFA-1 molecules. To address this question, we made use of the reporter antibody mAb24, which recognizes an activation epitope of the extracellular domains of the integrin (Figure 6). This epitope is up-regulated by either extracellular Mn2+ or the combination of Mg2+/EGTA (Dransfield et al., 1992). We discovered that overexpression of Ig–cytohesin-1 alone did not induce mAb24 epitope expression robustly (not shown). However, it was found that Ig–cytohesin-1 was capable of inducing binding of mAb24 in the presence of EGTA, and was thus partially substituting for extracellular Mg2+ (Figure 6B). Titration studies employing soluble ICAM-1–Fc chimeric protein revealed that half-maximal binding of this ligand to LFA-1 remained unaffected by Ig–cytohesin-1 expression, or mutant variants thereof (Figure 6C). This appears to be in agreement with a recently published study demonstrating that activation epitopes of LFA-1 do not necessarily correlate with high-affinity ligand binding (van Kooyk et al., 1999).
Fig. 6. Both cytohesin-1 and the guanine nucleotide exchange function-defective mutant cytohesin-1(E157K) induce the mAb24 epitope of LFA-1 in Jurkat cells. Half-maximal binding of the cells to soluble ICAM-1–Fc chimeric protein is not affected by overexpression of cytohesin-1, or mutant derivatives thereof. (A) Cytoplasmic Ig fusion proteins (as indicated) were expressed in Jurkat cells by recombinant vaccinia viruses, and the cells subsequently were fixed and permeabilized. Cytoplasmic expression of the chimeras is detected by an FITC-labeled, anti-human IgG antibody. The ‘control’ sample corresponds to uninfected cells. (B) mAb24 staining of aliquots from the cells shown in Figure 5A. Cells were incubated with 5 µg/ml mAb24 in HBSS in the presence of 2 mM EGTA. (C) Soluble ligand binding is not affected by cytohesin-1. The assay was carried out as described in Materials and methods. The input ICAM-1–Fc protein concentration is plotted versus the percentage of positive cells detected by flow cytometry.
Using the same assay, we went on to analyze whether the ARF exchange factor function of cytohesin-1 was required for cytohesin-1-dependent mAb24 epitope expression of LFA-1 (Figure 6B). To this end, we used a point mutant of cytohesin-1, cytohesin-1(E157K). The homologous residue of the Sec7 domain of ARNO (cytohesin-2), E156, had been determined by both structural and functional analysis to be critical for catalytic activity, and mutation of this amino acid into a lysine was found to abrogate GEF activity of cytohesin-2/ARNO completely. Accordingly, we observed a complete loss of GEF activity of cytohesin-1(E157K) towards recombinant ARF proteins in vitro (Knorr et al., 2000; not shown). When tested in the reporter epitope induction assay, we found that cytohesin-1(E157K) was functionally indistinguishable from cytohesin-1 in this experiment, i.e. it induced mAb24 binding. However, the PHc construct, which had been described before to inhibit Jurkat cell adhesion to ICAM-1, did not (Figure 6B). A likely interpretation of these data is that cytohesin-1 induces the mAb24 epitope by direct interaction with LFA-1, and that cytohesin-1(E157K) apparently retained this capacity.
The cytohesin-1(E157K) mutant abrogates T-cell adhesion to ICAM-1 and fails to induce cell spreading
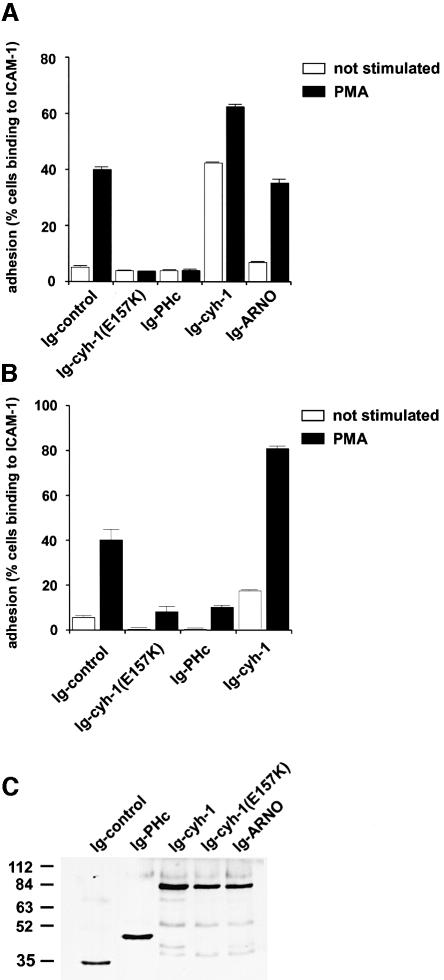
It has been debated whether inside-out signal transduction alone is sufficient for integrin-mediated cell adhesion. ‘Post-receptor events’, which include strong clustering of the receptors and cell spreading, have been shown to be important determinants for the induction of an adhesive phenotype (Lub et al., 1995; Peter and O’Toole, 1995; Stewart et al., 1998). Furthermore, such post-receptor events, which appear to involve the plasma membrane actin cytoskeleton, can be separated from affinity regulation mechanisms (Peter and O’Toole, 1995). We consequently performed in vitro adhesion assays (Figure 7) to address the question of whether the ARF exchange activity of cytohesin-1 was required for the regulation of LFA-1-dependent cell adhesion, because ARF GTPases had been implicated in the remodeling of the actin cytoskeleton (D’Souza et al., 1997; Norman et al., 1998; Franco et al., 1999). To this end, adhesion assays were performed with Jurkat cells and with PBLs, using an immobilized ICAM–Fc chimera as a ligand (Kolanus et al., 1996). Recombinant vaccinia viruses (Kolanus et al., 1996) mediated expression of the respective cytohesin-1 or cytohesin-1 subdomain constructs, and adhesion was monitored with or without the inclusion of an exogenous stimulus, namely 40 ng/ml PMA. Figure 7 shows that adhesion of both Jurkat cells (Figure 7A) and human PBLs (Figure 7B) infected with recombinant vaccinia viruses expressing cytohesin-1(E157K) was strongly inhibited. Overexpression of the cytohesin-1 point mutant had essentially the same inhibitory effect as the C-terminal module, comprising the PH domain and polybasic region (Ig–PHc, Figure 7), previously described to interfere with membrane recruitment of endogenous cytohesin-1.
Fig. 7. Stimulated adhesion of Jurkat E6 cells or human peripheral blood lymphocytes to ICAM-1 is abrogated by the guanine nucleotide exchange function-defective mutant cytohesin-1(E157K). Cytoplasmic Ig fusion constructs were expressed by recombinant vaccinia viruses in the respective cells, and the adhesion assay was performed as described in Materials and methods. (A) Adhesion of Jurkat E6 cells. (B) Adhesion of peripheral blood lymphocytes. (C) Immunoblot analysis showing expression of selected Ig fusions used in this experiment.
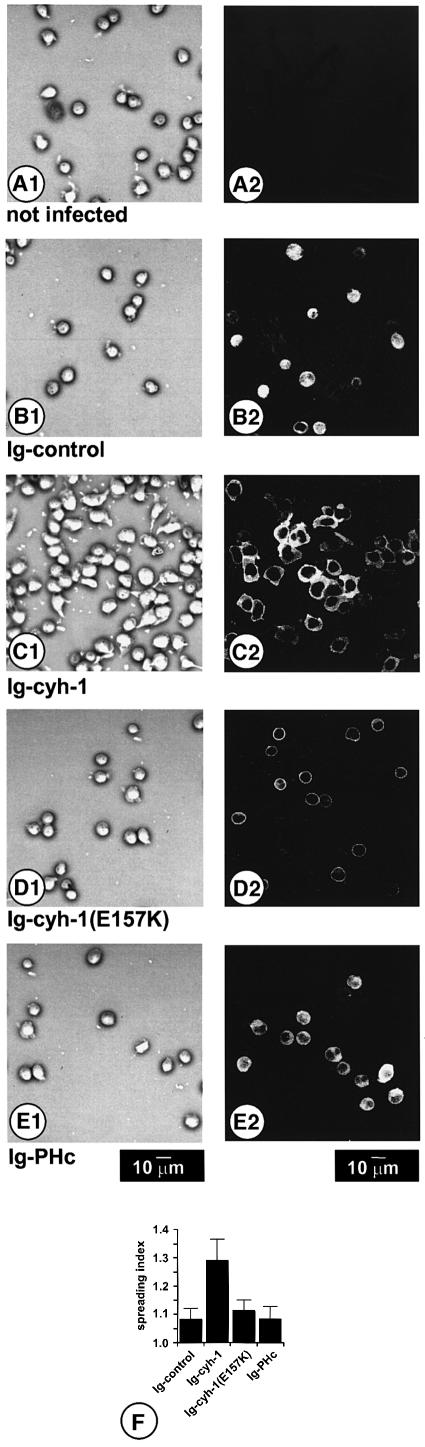
Since we had observed before that overexpression of cytohesin-1 induced strong spreading of Jurkat cells on ICAM-1, but not on unspecific substrates such as poly-l-lysine (Nagel et al., 1998a; W.Kolanus, unpublished observations), we analyzed the effects of cytohesin-1 (E157K) overexpression on the ability of Jurkat cells to spread on ICAM-1. Phase contrast microscopy (Figure 8, left panel, A1–E1) revealed that expression of cytohesin-1 (C1) induced strong spreading of Jurkat cells on ICAM-1, whereas expression of cytohesin-1(E157K) (D1) or of the PH domain/polybasic sequence module (PHc) (E1) did not result in spreading. Using the NIH image analysis software, we found that approximate quantification of the surface area occupied by the adherent cells correlated well with the qualitative impression, revealing the highest spreading index for Ig–cytohesin-1-expressing cells (Figure 8). The failure of the dominant-negative cytohesin-1 constructs to induce spreading of Jurkat cells is correlated directly with their ability to interfere with cell adhesion. Expression controls (anti-Ig stainings, Figure 8, right panel, A2–E2) showed that the respective constructs were expressed in almost all cells.
Fig. 8. Cytohesin-1 induces spreading of Jurkat E6 cells on ICAM-1. At 8 h post-infection with recombinant vaccinia viruses, Jurkat E6 cells were plated on ICAM-1 for 20 min; non-adherent cells were washed off with HBSS. After fixation of adherent cells, phase-contrast micrographs (first panel, A1, B1, C1, D1 and E1) were taken. For subsequent laser-scanning microscopical analysis (second panel, A2, B2, C2, D2 and E2), Ig fusion proteins were stained with an FITC-labeled anti-Ig antibody. Expression of wild-type Ig–cytohesin-1 (C1 and C2) causes strong spreading of cells on ICAM-1. In contrast, cells do not spread when they express cytohesin-1(E157K) (D1 and D2), Ig–PHc (E1 and E2) or a control construct (B1 and B2), or if they are not infected (A1 and A2). In all these cases, cells retain a spherical shape, and thus do not spread on ICAM-1. The spreading index (F) of 50 cells per field was quantified as described in Materials and methods.
Taken together, our data indicate that induction of the mAb24 epitope of LFA-1 by cytohesin-1 or cytohesin-1(E157K) does not correlate with the effects of these proteins seen on cell adhesion and spreading: cytohesin-1(E157K), which strongly abrogates Jurkat cell adhesion and spreading, retains the ability to induce the mAb24 epitope of LFA-1. It had been described by others that expression of extracellular activation epitopes and robust cell adhesion can be separated at the molecular level and that two distinct regions of the β-2 cytoplasmic domain were important in this (Peter and O’Toole, 1995). Our data suggest that both aspects may nonetheless be regulated by two separable functions of cytohesin-1.
Discussion
In this study, we provide evidence for a dual mechanistic role of cytohesin-1 in the regulation of LFA-1–ICAM-1 interaction and in cell spreading. Cytohesin-1 initially was characterized as a β-2 integrin-binding protein, but it had also been shown to be a member of a growing family of GEFs for ARF G-proteins. Here we demonstrate that both activities appear to be required for its function as an LFA-1-regulatory molecule, and the mechanistic implications resulting from our observations will be discussed.
Our data indicate a strong physical and functional connection between LFA-1 and cytohesin-1. We show here, with the help of a cytohesin-1-specific monoclonal antibody, that endogenous LFA-1 and cytohesin-1 strongly co-localize at the plasma membrane in a human lymphoblastoid B-cell line. Furthermore, immunoblot analyses revealed that cytohesin-1 invariably shows strong expression in cells which express high levels of LFA-1.
Mutational analyses revealed that inactivation of the cytohesin-1-binding site in the β-2 cytoplasmic domain results in a strong activation defect of the integrin. Furthermore, the mutant no longer responds to cytohesin-1 overexpression. This was clearly not due to gross structural alterations because the mutant LFA-1 molecule can still be activated by divalent cations. The cytohesin-1-interacting region in CD18 comprises the membrane-proximal segment of the β-2 cytoplasmic domain, and a similar sequence element previously had been implicated in affinity regulation of β-1 integrins (Hughes et al., 1995). Thus, it appears that the interaction of LFA-1 with cytohesin-1 is an important determinant of the activation potential of the integrin, unless intracellular regulatory mechanisms are bypassed by high, non-physiological concentrations of extracellular divalent cations.
It is shown in Figure 6 that cytohesin-1 overexpression results in partial induction of the mAb24 epitope in the presence of exogenous EGTA. Most significantly, both cytohesin-1 and cytohesin-1(E157K) contribute to the enhanced binding of mAb24. This finding implies that the GEF function of cytohesin-1 is not required for induction of this epitope, although it is most important for adhesion and spreading (discussed below). Furthermore, our binding data clearly rule out that E157K interferes with binding of cytohesin-1 to CD18. Thus, expression of activation epitopes and LFA-1-mediated cell adhesion may be uncoupled, but both appear to be regulated through differential functions of one and the same cytoplasmic regulatory molecule, cytohesin-1. Soluble, dimeric ligand binding appeared not to be altered by the cytohesin-1 pathway. This would favor the assumption that cytohesin-1 enhances clustering of LFA-1 at some level. Overexpression of the molecule alone does not result in a dramatic clustering of the integrin (data not shown), which had been observed when LFA-1 cytoplasmic domain mutants were analyzed in previous studies (Lub et al., 1997a; van Kooyk et al., 1999). However, oligomerization events may not necessarily be detectable at the microscopic level.
We have tested further whether the ARF GDP–GTP exchange function is a requirement for the role of cytohesin-1 in cell adhesion. It was found that the introduction of the E157K mutation, which abrogates all GEF activity of cytohesin-1 towards ARFs in vitro, acts as a dominant-negative inhibitor of LFA-1-mediated cell binding to ICAM-1 following stimulation by PMA. This finding clearly implicates the GEF function of cytohesin-1 in the regulation of LFA-1-mediated cell adhesion. What is the mechanistic role of the exchange factor in this context? In the light of the known functions of ARF proteins in eukaryotic cells, two possibilities immediately come to mind: intracellular vesicle transport and/or remodeling of the plasma membrane actin cytoskeleton. Vesicle transport mechanisms appear to regulate the vectorial cycling of integrins from the trailing to the leading edge of migrating neutrophils (Lawson and Maxfield, 1995), and have therefore been implicated in biological integrin functions pertaining to the subject discussed here, because dynamic affinity regulation of integrins is an important aspect of cell migration on integrin ligands (Palecek et al., 1997). On the other hand, both ARF1 and ARF6 functions have been shown to have an impact on specific events in the regulation of the plasma membrane actin cytoskeleton, such as, for example, paxilin deposition at focal contacts (Norman et al., 1998) or induction of membrane ruffling (Radhakrishna et al., 1999). Based on our studies here, we cannot favor or rule out either of these functions, which might also be linked and coordinated (Critchley et al., 1999). However, cell spreading, which is clearly abrogated by introduction of the E157K mutation, is probably linked to actin remodeling events (Lub et al., 1997a), and we have observed, furthermore, changes in the composition of cellular F-actin when cytohesin-1 or E157K are expressed in Jurkat cells (W.Nagel and W.Kolanus, in preparation). Our data presented here do not allow us to address the relationship of the cytohesin-1 pathway to more distal functions in the actin cytoskeleton such as a potential calpain–protease-dependent mechanism (Stewart et al., 1998) or the enhancement of the lateral mobility of LFA-1 (Dustin, 1998). However, our findings certainly are compatible with these concepts.
Another important point, which arises in this context, is the identity of the cellular ARF target of cytohesin-1. We have tested dominant-negative and constitutively active forms of both ARF1 and ARF6 in adhesion assays (not shown) and found that neither factor affected LFA-1-mediated adhesion to ICAM-1. Of course this leaves open the possibility of the involvement of other known ARFs or ARF-like proteins, and these need to be tested in the future. However, it is possible that unidentified ARFs or ARF-like factors serve as cellular targets for cytohesin-1. A high degree of specificity in this system is documented by the fact that overexpression of cytohesin-2/ARNO does not induce cell adhesion in our experiments. It seems plausible, therefore, that cytohesin-1, a protein which is found predominantly in hematopoietic cells, targets a specific cellular ARF protein. Other small G-proteins cannot be ruled out fully as candidates, but structural considerations make this an unlikely hypothesis (Goldberg, 1998; Mossessova et al., 1998).
Taken together, our study provides strong evidence that cytohesin-1 acts as an important cytoplasmic regulator of LFA-1 activation and LFA-1-dependent spreading of lymphoid cells on ICAM-1. This activity is provided by two independent and separable subfunctions: by molecular interaction of the regulatory molecule with the integrin and by its GEF activity.
Materials and methods
Generation of a cytohesin-1-specific monoclonal antibody
Rat monoclonal antibodies were raised against the prokaryotic fusion protein encoded by His6-cytohesin-1 expression plasmid (Kolanus et al., 1996) according to Kremmer et al. (1997), with modifications: Lou/C rats were immunized twice with 50 µg of purified recombinant His6-cytohesin-1 and boostered once with the same amount of protein. Spleen cells were fused to the myeloma cell line P3X63Ag8.653 according to standard protocols (Kohler and Milstein, 1975). Hybridoma supernatants were screened for antibodies by enzyme-linked immunosorbent assay (ELISA). Hybridomas secreting antibodies against purified His6-cytohesin-1 were cloned twice. Among the various clones tested, the monoclonal antibody mAb7H2 was selected.
Microscopy
721-LCL cells were incubated with either anti-CD18 mouse monoclonal antibody KIM185 or mouse anti-CD29 antibody 4B7R (Ancell, Bayport, MN) at 1 µg/ml for 10 min, washed with Hank’s buffered salt solution (HBSS) followed by cross-linking with secondary goat anti-mouse Texas red-conjugated F(ab′)2 fragment at 37°C for 5 min. After a final wash with HBSS, cells were placed on poly-l-lysine-coated glass coverslips (Marienfeld, Germany) for 15 min at 37°C in a humidified chamber. Adhered cells were fixed with freshly prepared 2% (w/v) paraformaldehyde in phosphate-buffered saline (PBS) for 1 h at 4°C, and blocked with 2% (w/v) glycine in PBS for 2 h. Subsequently, cells were permeabilized for 10 min with 0.2% (v/v) Triton X-100 in PBS. For detection of endogenous cytohesin-1, cells were incubated twice with hybridoma supernatants containing mAb7H2. mAb7H2 binding was detected by a fluorescein-conjugated F(ab′)2 fragment of affinity-purified goat anti-rat IgG, bearing minimal cross-reactivity to mouse serum proteins. After the final wash with PBS, slides were mounted on mounting medium (Vector Laboratories, Burlingame, CA) and samples were examined by laser scanning microscopy (Leica TCS-NT system, Leica). Confocal images were collected as 512×512 pixel files and processed with the help of the Photoshop program (Adobe).
Quantification of cell spreading
Quantification of cell morphology was performed as described previously (Woodside et al., 1998). Images of cells adhered to ICAM-1 were digitized and analyzed with NIH Image software (available from ftp:/codon.nih.gov pub/nih-image/nih-image 161_fat.hqx). The deviation of each cell from perfect roundness was calculated using a formula that divided the theoretical maximum area for a given perimeter (perimeter2/4Π) by the observed pixel area. The value for a perfectly round cell equals 1.0, and a larger value represents the spreading index >1.0. Random fields of adherent cells were captured and the spreading index was quantified.
In vitro protein ‘pull-down’ assay
Prior to the in vitro pull-down, 96-well microtiter plates (Falcon) were coated with a hybridoma supernatant containing mAb7H2, directed against cytohesin-1, for 90 min at 25°C and blocked with 1% (w/v) bovine serum albumin (BSA) in PBS for 90 min at 25°C. The plates were then incubated with recombinant polyhistidine-tagged cytohesin-1 or cytohesin-1 (E157K), purified from E.coli and diluted in lysis buffer (at 10 ng/µl) on ice for 4 h. COS cells were infected with recombinant vaccinia viruses (Kolanus et al., 1993, 1996), directing expression of CD18 fusion proteins. Infected cells subsequently were incubated in full medium (RPMI/10% v/v fetal bovine serum) for 14 h. Cells were lysed in buffer containing 100 mM Tris pH 7.5, 150 mM NaCl, 1% (v/v) Brij58 (Pierce), 10 µg/ml leupeptin, 10 µg/ml aprotinin and 1 mM phenylmethylsulfonyl fluoride. Insoluble material was removed by centrifugation and the supernatant was transferred to the microtiter plates. Incubation was continued on ice for 90 min. Following several washes with lysis buffer, the contents of four wells were combined by collection with SDS sample buffer (6 µl/well), and analyzed by SDS–PAGE (12%) and standard Western blotting techniques.
Flow cytometry
Cells were infected with recombinant vaccinia virus, incubated in full medium for several hours, collected by centrifugation and washed with PBS. For extracellular staining, cells were incubated with antibody directed against the protein of interest on ice for 1 h in PBS, washed once with ice-cold PBS and incubated with a fluorescein isothiocyanate (FITC)-conjugated secondary antibody on ice for another 60 min. Fluorescence measurement was then carried out on a Coulter® EPICS XL-MCL. mAb24 staining was performed in a similar fashion, but for these experiments cells were incubated in HBSS containing 2 mM EGTA, employing 5 µg/ml mAb24. The cells subsequently were stained with a secondary reagent, an affinity-purified FITC-labeled anti-mouse IgG (Dianova, Hamburg).
Yeast two-hybrid system
‘Bait’ proteins were fused at their N-termini to the DNA-binding domain of the LexA-repressor, using the pEG202 vector (Gyuris et al., 1993; Zervos et al., 1993). ‘Preys’ were constructed by fusing the respective interaction elements to the C-terminus of the activation domain B42, employing the pJG4-5 vector system (Gyuris et al., 1993; Zervos et al., 1993). For the interaction studies, double transformations into yeast strain EGY48 bearing the JK103 lacZ reporter plasmid were performed and growth of yeast cells was characterized for their ability to grow on medium lacking leucine. Interaction of bait and prey protein was monitored by assessing lacZ gene activity (blue color of the yeast colonies) in the presence of the inducer galactose and the reporter dye X-gal (Gyuris et al., 1993; Zervos et al., 1993).
Adhesion assay
Cells (Jurkat, HeLa, LAD19 and SK-β2.7) were infected with recombinant vaccinia virus, and measurement of cell adhesion to an ICAM-1–Fc fusion protein was carried out as described previously (Kolanus et al., 1996; Nagel et al., 1998a,b) with the following modifications: vaccinia virus infection of HeLa cells was performed for 12 h. Stimulation of cells with PMA (Sigma) at 40 ng/ml or with anti-TCR antibody OKT3 at 2 µg/ml was performed at 37°C for 30 min. The results represent an average of at least three independent experiments carried out in quadruplicate for each value.
Soluble ligand-binding assay
The ICAM-1–Fc fusion protein was purified from COS cell supernatants by binding to protein A–Sepharose beads (Kolanus et al., 1996), and the soluble ligand-binding assay was carried out essentially as described before (van Kooyk et al., 1999). Briefly, vaccinia virus-infected Jurkat cells were resuspended in TSA [20 mM Tris–HCl pH 8.0, 150 mM NaCl, 1 mM CaCl2, 2 mM MgCl2, 0.5% (w/v) BSA]. A total of 50 000 cells subsequently were incubated with various concentrations of purified soluble ICAM-1–Fc for 30 min at 37°C, using 96-well V-shaped bottom plates. Cells were then washed with TSA and incubated for 15 min at 37°C with an FITC-conjugated goat anti-human Fc-specific antibody (Jackson Immunoresearch Laboratories, West Grove, PA). Cells subsequently were washed and resuspended in 100 µl of TSA. Binding of the reagents to cells was measured by flow cytometry, using a Coulter Epic-XL flow cytometer. Background binding was determined by using a preparation of human IgG1 as a control. Although usually negligible, background fluorescence was subtracted from the given values if necessary.
Constructs
PCR amplification of the CD18 cytoplasmic domain was performed as described (Kolanus et al., 1996). For the various deletion mutants, the following PCR primers were used:
GCGGGGGCGGCCGCTTTAAAGGGGATTATCATTGTTCC (CD18cyt Δ754–769),
GCGGGGGCGGCCGCTTTACCACTGGGACTTGAGC (CD18cytΔ748–769),
GCGGGGGCGGCCGCTTTACTCAAAGCGCCTGTAC (CD18cytΔ740–769),
GCGGGGGCGGCCGCTTTAGTCGCTCAGGTGGATC (CD18cytΔ732–769),
GCGGGGACGCGTCTCCGGGAGTACAGGCGCTTT,
GCGGGGGCGGCCGCTTTAACTCTCAGC (CD18cytΔ723–731)
and GTACTCCCTGAGGTCGCTCAGGTGGATCAGACCCTTCCA,
GCGGGGGCGGCCGCTTTAACTCTCAGC
(CD18cytWKA723–725TRG).
The resulting products were inserted into the pEG202 yeast expression vector.
For cloning of transmembrane Ig–CD18 fusion plasmids bearing the transmembrane and cytoplasmic domain of CD18 (sIg–CD18tm/cyt), the original sIg–CD7 transmembrane domain (Zeitlmann et al., 1998) was substituted by the corresponding transmembrane sequence of CD18 followed by the CD18 intracellular portion. For PCR amplification of these constructs, the primers GCGGGGGGATCCAGCAGGCCCCA ACATCGCCGC, GCGGGGGCGGCCGCTTTAACTCTCAGC (wild-type) and GCGGGGGGATCCAGCAGGCCCCAACATCGCCGC, GATGACCAGCAGGAGAATGCCGATCAGC, CTCCTGCTGGTCA TCACGCGTGGTCTGATCCAC, GCGGGGGCGGCCGCTTTAACT CTCAGC (mutant WKA723–725TRG) were used and subcloned into the BamHI and NotI sites of the psIgTKG vaccinia expression vector. PsIgTKG is a derivative of the pTKG expression vector (Romeo et al., 1992) containing the transmembrane Ig fusion protein (sIg) sequence.
The CD18 chain, containing the consecutive triple mutation WKA723–725TRG, was amplified by two-step PCR using the primers GCGGGGGCTCAGCGTGCCAGTGCGAGAGGACCACTGAGG, GC GGGGACGCGTGATGACCAGCAGGAGAATGCC and GCGGGG ACGCGTGGTCTGATCCACCTGAGCGACCTCCGG, GCGGGGGC GGCCGCTTTAACTCTCAGC and inserted into the pTKG (CD18) vector.
Establishment of HeLa cell lines stably expressing LFA-1 wild-type and mutant forms
CD18 wt, CD18 WKA723–725TRG and CD11a cDNA, respectively, were subcloned into the vector pEF-IRES-puro, which contained a bicistronic expression cassette transcribed from the human EF.1α promoter (L.Zeitlmann and W.Kolanus, unpublished data). The corresponding LFA-1 chains were electroporated into HeLa cells and cells stably expressing LFA-1 were isolated subsequently by puromycin selection (Biomol) at a concentration of 2 µg/ml. Expression of both CD18 and CD11a was analyzed by flow cytometry as described above.
Acknowledgments
Acknowledgements
We would like to thank E.-L.Winnacker and R.Grosschedl for continuing support, and members of the laboratory for discussion and advice. We are particularly grateful to Vaclav Horejsi, Rik Bleijs and Martyn Robinson for the kind donation of reagents, and to Pierre Schilcher for excellent technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft and by the Wilhelm-Sander Stiftung.
References
- Carr M.W., Alon,R. and Springer,T.A. (1996) The C-C chemokine MCP-1 differentially modulates the avidity of β1 and β2 integrins on T lymphocytes. Immunity, 4, 179–187. [DOI] [PubMed] [Google Scholar]
- Chan A.S., Mobley,J.L., Fields,G.B. and Shimizu,Y. (1997) CD7-mediated regulation of integrin adhesiveness on human T cells involves tyrosine phosphorylation-dependent activation of phosphatidylinositol 3-kinase. J. Immunol., 159, 934–942. [PubMed] [Google Scholar]
- Critchley D.R., Holt,M.R., Barry,S.T., Priddle,H., Hemmings,L. and Norman,J. (1999) Integrin-mediated cell adhesion: the cytoskeletal connection. Biochem. Soc. Symp., 65, 79–99. [PubMed] [Google Scholar]
- D’Souza S.C., Boshans,R.L., McDonough,M., Stahl,P.D. and Van,A.L. (1997) A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements. EMBO J., 16, 5445–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M.S. and Springer,T.A. (1994) The dynamic regulation of integrin adhesiveness. Curr. Biol., 4, 506–517. [DOI] [PubMed] [Google Scholar]
- Dransfield I., Cabanas,C., Craig,A. and Hogg,N. (1992) Divalent cation regulation of the function of the leukocyte integrin LFA-1. J. Cell Biol., 116, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Saido,T.C., Tsubuki,S., Indig,F.E., Williams,M.J. and Ginsberg,M.H. (1995) Calpain cleavage of the cytoplasmic domain of the integrin β3 subunit. J. Biol. Chem., 270, 26146–26151. [DOI] [PubMed] [Google Scholar]
- Dustin M.L. (1998) Making a little affinity go a long way: a topological view of LFA-1 regulation. Cell Adhes. Commun., 6, 255–262. [DOI] [PubMed] [Google Scholar]
- Dustin M.L. and Springer,T.A. (1989) T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature, 341, 619–624. [DOI] [PubMed] [Google Scholar]
- Fields S. and Song,O. (1989) A novel genetic system to detect protein–protein interactions. Nature, 340, 245–246. [DOI] [PubMed] [Google Scholar]
- Fox J.E., Taylor,R.G., Taffarel,M., Boyles,J.K. and Goll,D.E. (1993) Evidence that activation of platelet calpain is induced as a consequence of binding of adhesive ligand to the integrin, glycoprotein IIb–IIIa. J. Cell Biol., 120, 1501–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M., Peters,P.J., Boretto,J., van,D.E., Neri,A., D’Souza,S.C. and Chavrier,P. (1999) EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J., 18, 1480–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S.R., Hatfield,J.C. and Casanova,J.E. (1998) Remodeling of the actin cytoskeleton is coordinately regulated by protein kinase C and the ADP-ribosylation factor nucleotide exchange factor ARNO. Mol. Biol. Cell, 9, 3133–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. (1998) Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell, 95, 237–248. [DOI] [PubMed] [Google Scholar]
- Grotewiel M.S., Beck,C.D., Wu,K.H., Zhu,X.R. and Davis,R.L. (1998) Integrin-mediated short-term memory in Drosophila. Nature, 391, 455–460. [DOI] [PubMed] [Google Scholar]
- Gyuris J., Golemis,E., Chertkov,H. and Brent,R. (1993) Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell, 75, 791–803. [DOI] [PubMed] [Google Scholar]
- Hemler M.E. (1998) Integrin associated proteins. Curr. Opin. Cell Biol., 10, 578–585. [DOI] [PubMed] [Google Scholar]
- Hmama Z., Knutson,K.L., Herrera,V.P., Nandan,D. and Reiner,N.E. (1999) Monocyte adherence induced by lipopolysaccharide involves CD14, LFA-1 and cytohesin-1. Regulation by Rho and phosphatidylinositol 3-kinase. J. Biol. Chem., 274, 1050–1057. [DOI] [PubMed] [Google Scholar]
- Huang Y. and Wu,C. (1999) Integrin-linked kinase and associated proteins. Int. J. Mol. Med., 3, 563–572. [DOI] [PubMed] [Google Scholar]
- Hughes P.E. and Pfaff,M. (1998) Integrin affinity modulation. Trends Cell Biol., 8, 359–364. [DOI] [PubMed] [Google Scholar]
- Hughes P.E., O’Toole,T.E., Ylanne,J., Shattil,S.J. and Ginsberg,M.H. (1995) The conserved membrane-proximal region of an integrin cytoplasmic domain specifies ligand binding affinity. J. Biol. Chem., 270, 12411–12417. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. (1992) Integrins: versatility, modulation and signaling in cell adhesion. Cell, 69, 11–25. [DOI] [PubMed] [Google Scholar]
- Knorr T., Nagel,W. and Kolanus,W. (2000) Phosphoinositides determine specificity of the guanine-nucleotide exchange activity of cytohesin-1 for ADP-ribosylation factors derived from a mammalian expression system. Eur. J. Biochem., in press. [DOI] [PubMed] [Google Scholar]
- Kohler G. and Milstein,C. (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 256, 495–497. [DOI] [PubMed] [Google Scholar]
- Kolanus W. and Seed,B. (1997) Integrins and inside-out signal transduction: converging signals from PKC and PIP3. Curr. Opin. Cell Biol., 9, 725–731. [DOI] [PubMed] [Google Scholar]
- Kolanus W., Romeo,C. and Seed,B. (1993) T cell activation by clustered tyrosine kinases. Cell, 74, 171–183. [DOI] [PubMed] [Google Scholar]
- Kolanus W., Nagel,W., Schiller,B., Zeitlmann,L., Godar,S., Stockinger,H. and Seed,B. (1996) αLβ2 integrin/LFA-1 binding to ICAM-1 induced by cytohesin-1, a cytoplasmic regulatory molecule. Cell, 86, 233–242. [DOI] [PubMed] [Google Scholar]
- Kremmer E., Ohst,K., Kiefer,J., Brewis,N. and Walter,G. (1997) Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: abundant expression of both forms in cells. Mol. Cell. Biol., 17, 1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson M.A. and Maxfield,F.R. (1995) Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature, 377, 75–79. [DOI] [PubMed] [Google Scholar]
- Lub M., van Kooyk,Y. and Figdor,C.F. (1995) Ins and outs of LFA-1. Immunol. Today, 16, 479–483. [DOI] [PubMed] [Google Scholar]
- Lub M., van Kooyk,Y., van Vliet,S. and Figdor,C.G. (1997a) Dual role of the actin cytoskeleton in regulating cell adhesion mediated by the integrin lymphocyte function-associated molecule-1. Mol. Biol. Cell, 8, 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lub M., van Vliet,S., Oomen,S.P., Pieters,R.A., Robinson,M., Figdor, C.G. and van Kooyk,Y. (1997b) Cytoplasmic tails of β1, β2 and β7 integrins differentially regulate LFA-1 function in K562 cells. Mol. Biol. Cell, 8, 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacci E., Tsai,S.C., Adamik,R., Moss,J. and Vaughan,M. (1997) Cytohesin-1, a cytosolic guanine nucleotide-exchange protein for ADP-ribosylation factor. Proc. Natl Acad. Sci. USA, 94, 1745–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J. and Vaughan,M. (1998) Molecules in the ARF orbit. J. Biol. Chem., 273, 21431–21434. [DOI] [PubMed] [Google Scholar]
- Mossessova E., Gulbis,J.M. and Goldberg,J. (1998) Structure of the guanine nucleotide exchange factor Sec7 domain of human ARNO and analysis of the interaction with ARF GTPase. Cell, 92, 415–423. [DOI] [PubMed] [Google Scholar]
- Nagel W., Schilcher,P., Zeitlmann,L. and Kolanus,W. (1998a) The PH domain and the polybasic C domain of cytohesin-1 cooperate specifically in plasma membrane association and cellular function. Mol. Biol. Cell, 9, 1981–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel W., Zeitlmann,L., Schilcher,P., Geiger,C., Kolanus,J. and Kolanus,W. (1998b) Phosphoinositide 3-OH kinase activates the β2 integrin adhesion pathway and induces membrane recruitment of cytohesin-1. J. Biol. Chem., 273, 14853–14861. [DOI] [PubMed] [Google Scholar]
- Nielsen M., Svejgaard,A., Skov,S., Dobson,P., Bendtzen,K., Geisler,C. and Odum,N. (1996) IL-2 induces β2-integrin adhesion via a wortmannin/LY294002-sensitive, rapamycin-resistant pathway. Phosphorylation of a 125-kilodalton protein correlates with induction of adhesion, but not mitogenesis. J. Immunol., 157, 5350–5358. [PubMed] [Google Scholar]
- Norman J.C., Jones,D., Barry,S.T., Holt,M.R., Cockcroft,S. and Critchley,D.R. (1998) ARF1 mediates paxillin recruitment to focal adhesions and potentiates Rho-stimulated stress fiber formation in intact and permeabilized Swiss 3T3 fibroblasts. J. Cell Biol., 143, 1981–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek S.P., Loftus,J.C., Ginsberg,M.H., Lauffenburger,D.A. and Horwitz,A.F. (1997) Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature, 385, 537–540. [DOI] [PubMed] [Google Scholar]
- Peter K. and O’Toole,T.E. (1995) Modulation of cell adhesion by changes in αLβ2 (LFA-1, CD11a/CD18) cytoplasmic domain/cytoskeleton interaction. J. Exp. Med., 181, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H., Al,A.O., Khachikian,Z. and Donaldson,J.G. (1999) ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J. Cell Sci., 112, 855–866. [DOI] [PubMed] [Google Scholar]
- Romeo C., Kolanus,W., Amiot,M. and Seed,B. (1992) Activation of immune system effector function by T-cell or Fc receptor intracellular domains. Cold Spring Harb. Symp. Quant. Biol., 57, 117–125. [DOI] [PubMed] [Google Scholar]
- Sastry S.K. and Horwitz,A.F. (1993) Integrin cytoplasmic domains: mediators of cytoskeletal linkages and extra- and intracellular initiated transmembrane signaling. Curr. Opin. Cell Biol., 5, 819–831. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Mobley,J.L., Finkelstein,L.D. and Chan,A.S. (1995) A role for phosphatidylinositol 3-kinase in the regulation of β1 integrin activity by the CD2 antigen. J. Cell Biol., 131, 867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M.P., McDowall,A. and Hogg,N. (1998) LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J. Cell Biol., 140, 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooyk Y., van de Wiel-van-Kemenade,P., Weder,P., Kuijpers,T.W. and Figdor,C.G. (1989) Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature, 342, 811–813. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y., van Vliet,S.J. and Figdor,C.G. (1999) The actin cytoskeleton regulates LFA-1 ligand binding through avidity rather than affinity changes. J. Biol. Chem., 274, 26869–26877. [DOI] [PubMed] [Google Scholar]
- Venkateswarlu K., Gunn,M.F., Tavar,J.M. and Cullen,P.J. (1999) EGF- and NGF-stimulated translocation of cytohesin-1 to the plasma membrane of PC12 cells requires PI 3-kinase activation and a functional cytohesin-1 PH domain. J. Cell Sci., 112, 1957–1965. [DOI] [PubMed] [Google Scholar]
- Vennegoor C.J., van de Wiel-van Kemenade,E., Huijbens,R.J., Sanchez-Madrid,F., Melief,C.J. and Figdor,C. (1992) Role of LFA-1 and VLA-4 in the adhesion of cloned normal and LFA-1 (CD11/CD18)-deficient T cells to cultured endothelial cells. Indication for a new adhesion pathway. J. Immunol., 148, 1093–1101. [PubMed] [Google Scholar]
- Weber K.S., York,M.R., Springer,T.A. and Klickstein,L.B. (1997) Characterization of lymphocyte function-associated antigen 1 (LFA-1)-deficient T cell lines: the αL and β2 subunits are interdependent for cell surface expression. J. Immunol., 158, 273–279. [PubMed] [Google Scholar]
- Woodside D.G., Wooten,D.K. and McIntyre,B.W. (1998) Adenosine diphosphate (ADP)-ribosylation of the guanosine triphosphatase (GTPase) rho in resting peripheral blood human T lymphocytes results in pseudopodial extension and the inhibition of T cell activation. J. Exp. Med., 188, 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlmann L., Knorr,T., Knoll,M., Romeo,C., Sirim,P. and Kolanus,W. (1998) T cell activation induced by novel gain-of-function mutants of Syk and ZAP-70. J. Biol. Chem., 273, 15445–15452. [DOI] [PubMed] [Google Scholar]
- Zell T., Hunt,S.R., Mobley,J.L., Finkelstein,L.D. and Shimizu,Y. (1996) CD28-mediated up-regulation of β1-integrin adhesion involves phosphatidylinositol 3-kinase. J. Immunol., 156, 883–886. [PubMed] [Google Scholar]
- Zervos A.S., Gyuris,J. and Brent,R. (1993) Mxi1, a protein that specifically interacts with Max to bind Myc–Max recognition sites. Cell, 72, 223–232. [DOI] [PubMed] [Google Scholar]