Abstract
Peripheral and central thermoreceptors are involved in sensing ambient and body temperature, respectively. Specialized cold and warm receptors are present in dorsal root ganglion sensory fibres as well as in the anterior/preoptic hypothalamus. The two-pore domain mechano-gated K+ channel TREK-1 is highly expressed within these areas. Moreover, TREK-1 is opened gradually and reversibly by heat. A 10°C rise enhances TREK-1 current amplitude by ∼7-fold. Prostaglandin E2 and cAMP, which are strong sensitizers of peripheral and central thermoreceptors, reverse the thermal opening of TREK-1 via protein kinase A-mediated phosphorylation of Ser333. Expression of TREK-1 in peripheral sensory neurons as well as in central hypothalamic neurons makes this K+ channel an ideal candidate as a physiological thermoreceptor.
Keywords: DRG neurons/hypothalamus/2P domain K+ channels/thermoreceptors
Introduction
Sensing of ambient temperature is mediated almost exclusively by cutaneous thermoreceptors (Darian-Smith and Johnson, 1977; Darian-Smith, 1984; Spray, 1986). These warm and cold receptors allow the detection of rapid changes in skin temperature. However, the central neural mechanisms of thermal regulation involve mainly the hypothalamus, which controls body heat loss, heat retention and heat production (Boulant, 1998a,b).
Recent progress has been made in the molecular identification of peripheral heat detectors (Caterina and Julius, 1999; Cesare et al., 1999; Kress and Zeilhofer, 1999; Nagy and Rang, 1999a). Responses to painful heat involve the opening of the capsaicin receptor VR1 and its homologue VRL-1 in dorsal root ganglion (DRG) nociceptive neurons (Caterina et al., 1997, 1999; Tominaga et al., 1998). Direct opening of the non-selective cationic channel VR1 by moderate thermal stimuli (43°C) in small diameter DRG sensory neurons induces depolarization and enhanced action potential discharge (Caterina et al., 1997). VR1 is opened similarly by protons and capsaicin, indicating that it may participate in the common detection of thermal, noxious and chemical stimuli in vivo (Tominaga et al., 1998). VRL-1 is a candidate for transducing high-threshold heat responses (52°C) in a subset of DRG medium to large diameter nociceptive neurons (Caterina et al., 1999). Recently, noxious heat has been shown to activate capsaicin-sensitive and also a subpopulation of capsaicin-insensitive DRG neurons. It was postulated that distinct molecular entities account for the membrane responses to heat and capsaicin (Nagy and Rang, 1999a,b).
Substantial progress has also been made in the understanding of central thermoregulation at the cellular level. In the warm-sensitive neurons of the preoptic and anterior hypothalamus, warming increases the rise of the prepotential and leads to enhanced action potential discharge (Kobayashi and Takahashi, 1993; Griffin and Boulant, 1995; Griffin et al., 1996). This stimulation has been attributed to a faster inactivation of A-type K+ channels in these neurons upon warming (Griffin et al., 1996).
Although the molecular basis of heat-sensitive neurons is beginning to be elucidated, knowledge about cold-sensitive neurons is still lacking. Peripheral sensory cold fibres discharge slowly at the resting skin temperature but evoke strong action potential discharge upon lowering the temperature (Darian-Smith and Johnson, 1977; Darian-Smith, 1984; Spray, 1986). Similarly, in the anterior and preoptic hypothalamus, cold-sensitive neurons respond to cooling by enhanced action potential firing (Boulant, 1998a,b). Specific cold sensory processes as well as specific combinations of common neuronal elements have been suggested to be involved in cold sensing function (Braun et al., 1980; Schaffer and Braun, 1992).
TREK-1 belongs to the novel family of mammalian two-pore (P) domain K+ channels with four transmembrane segments (TMS), 2P domains, an extended M1P1 external loop (60–70 residues) and with intracellular N- and C-termini (Fink et al., 1996, 1998; Lesage et al., 1996a; Duprat et al., 1997; Reyes et al., 1998; Salinas et al., 1999). TASK-1 encodes a background outward rectifier that is constitutively active at all voltages and inhibited by mild external acidosis near the physiological pH (Duprat et al., 1997). TREK-1 is an outward rectifier mechanosensitive K+ channel opened by membrane stretch, cell swelling and shear stress (Patel et al., 1998; Maingret et al., 1999b). Mechanical activation of TREK-1 is mimicked by polyunsaturated fatty acids such as arachidonic acid (AA), and by the anionic amphipath trinitrophenol (TNP) (Patel et al., 1998; Maingret et al., 1999b, 2000). TREK-1 is also opened by inhalational anaesthetics including chloroform, ether, halothane and isoflurane (Patel et al., 1999). Finally, intracellular acidosis in the absence of mechanical or chemical stimulation directly opens TREK-1 (Maingret et al., 1999b). Background K+ channels have been implicated in various important physiological functions including mechano, lipid, acid and possibly oxygen sensing in specialized cells (Fink et al., 1996, 1998; Buckler, 1997; Duprat et al., 1997; Patel et al., 1998, 1999; Maingret et al., 1999a,b, 2000; Lauritzen et al., 2000).
In the present report, we demonstrate that TREK-1 is opened reversibly by heat. The high expression of TREK-1 in peripheral DRG neurons, as well as in the central hypothalamic centres, suggests that this channel may play an important physiological role in temperature sensing. We discuss the possibility that TREK-1 may act as a cold sensor and thus might be a molecular component of the cold transduction pathway. At physiological temperature, the opening of TREK-1 would be polarizing, whereas at lower and cold temperatures TREK-1 would close and depolarize neurons, thus signalling cold information.
Results
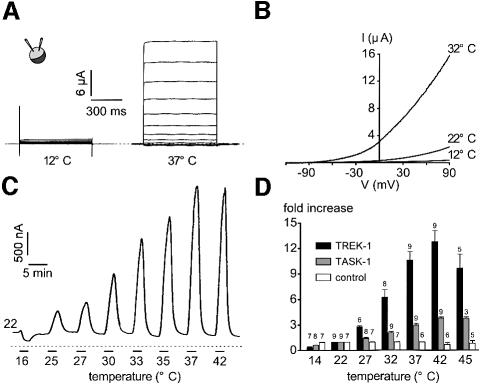
TREK-1 was expressed in Xenopus oocytes and channel activity was monitored using the two-microelectrode voltage-clamp technique. The current–voltage (I–V) curves shown in Figure 1A demonstrate that TREK-1 current is absent at 12°C and becomes strongly outwardly rectifying at 37°C. TREK-1 is time independent at all temperatures studied (Figure 1A). The reversal potential of the TREK-1 current induced by heat is –78 ± 3 mV (n = 12) in a physiological K+ gradient (Figure 1B). Lowering the temperature from 22 to 16°C suppresses the basal TREK-1 current measured at –20 mV (Figure 1C). On the contrary, a progressive rise in temperature induces a gradual and reversible strong activation of TREK-1 currents (Figure 1C and D). Temperatures >42°C lead to a non-reversible decrease in current amplitude (not shown), probably due to alteration of the oocytes at this high temperature (Figure 1D). The sensitivity to temperature is significantly higher for TREK-1 than for TASK-1 (Figure 1D). The increase in current amplitude for a temperature jump of 10°C is ∼7-fold for TREK-1 and 2-fold for TASK-1 in the range of 14–42°C (Figure 1D). The maximal temperature sensitivity of TREK-1 is observed between 32 and 37°C, with a 0.9-fold increase in current amplitude per degree Centigrade.
Fig. 1. TREK-1 is a temperature-sensitive K+ channel in Xenopus oocyte. (A) The two-microelectrode voltage-clamp technique was used to voltage-clamp oocytes expressing TREK-1. I–V curves of an oocyte expressing TREK-1 maintained at 12 and 37°C. The holding potential is –80 mV and increment voltage steps of 20 mV are applied every 5 s from –130 to 90 mV. (B) Voltage ramps of 800 ms duration applied from a holding potential of –80 mV are recorded at 12, 22 and 32°C in a TREK-1-expressing oocyte. (C) A TREK-1-expressing oocyte is voltage-clamped at a holding potential of –20 mV. The zero current is indicated by a dotted line. Cooling from 22 to 16°C inhibits TREK-1 basal activity while a gradual increase in temperature up to 42°C reversibly stimulates TREK-1 current amplitude. (D) Stimulation of current amplitude (fold increase It/I22°C) measured at –20 mV in control, TREK-1- and TASK-1-expressing oocytes. Note that at temperatures >42°C, TREK-1 is decreased irreversibly. At 22°C, current amplitude measured at –20 mV is 76 ± 3 nA (n = 7), 318 ± 45 nA (n = 9) and 1483 ± 197 nA (n = 9) for control, TREK-1- and TASK-1-expressing oocytes, respectively.
In transiently transfected COS cells, an increase in temperature from 22 to 42°C similarly potentiates TREK-1 currents (Figure 2A). Temperatures >42°C could not be tested as an important leak current develops at these temperatures. The reversal potential of the current induced by heat is –81.4 ± 1.3 mV (n = 5) (Figure 2A) in physiological K+ conditions and shifts to 1.1 ± 1.3 mV (n = 5) in symmetrical K+ conditions, demonstrating K+ selectivity. The stimulation by temperature is fast and completely reversible (see inset in Figure 2A). As observed in Xenopus oocytes, TREK-1 displays a higher sensitivity to temperature than TASK-1 when expressed in COS cells (Figure 2B). Deletion of the cytoplasmic N-terminal region of TREK-1 does not affect temperature stimulation (n = 3; not shown) but partial deletion of the C-terminal region of TREK-1 (Δ103) strongly reduces heat activation (Figure 2B). Similarly, a chimera containing the hydrophobic core of TREK-1 and the C-terminal region of TASK-1 (TR298/TA248) is only weakly sensitive to temperature (Figure 2B).
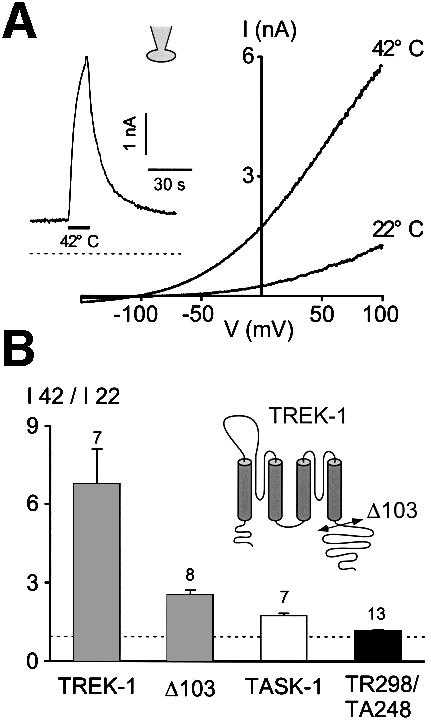
Fig. 2. TREK-1 is a heat-activated K+ channel in COS cells. (A) Voltage ramps of 800 ms duration are applied from a holding potential of –80 mV. The cell is bathed with an external medium containing 5 mM K+. Currents are recorded at 22 and 42°C, as indicated. The inset illustrates TREK-1 current induced by a temperature jump from 22 to 42°C (indicated by a horizontal bar) at a holding potential of 0 mV in physiological K+ conditions. Zero current is indicated by a dotted line. (B) Temperature sensitivity expressed as the ratio of current amplitude I42°C/I22°C measured at 0 mV of TREK-1, a C-terminally deleted TREK-1 mutant (Δ103), TASK-1 and a chimera containing the core of TREK-1 and the C-terminus of TASK-1 (TR298/TA248). Numbers of experiments are indicated. The cartoon illustrates the C-terminal deletion.
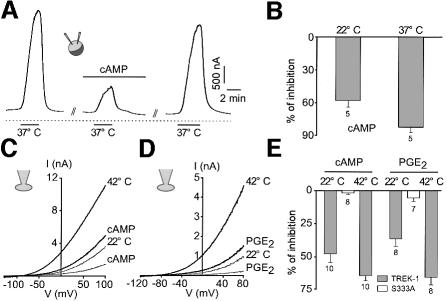
TREK-1 activation by heat is inhibited reversibly by cAMP in Xenopus oocytes as well as in COS cells (Figure 3). cAMP inhibition is slightly higher at increased temperatures and is fully reversible upon washing out (Figure 3A–C and E). In COS cells, but not in oocytes (n = 6), prostaglandin E2 (PGE2), activating endogenous COS prostaglandin receptors (E.Honoré and F.Maingret, unpublished observations), mimics the effect of cAMP and reversibly inhibits heat activation (Figure 3D and E). Again this effect is slightly enhanced at elevated temperature (Figure 3E). Substitution of Ser333 by alanine, in the consensus protein kinase A (PKA) phosphorylation site located in the C-terminal region of TREK-1, suppresses both cAMP and PGE2 inhibition (Figure 3E).
Fig. 3. cAMP and PGE2 reverse thermal stimulation of TREK-1. (A) Effect of 0.5 mM CPT-cAMP on TREK-1 current evoked by a temperature jump from 22 to 37°C in Xenopus oocytes. The current is measured at a holding potential of –20 mV. cAMP is applied for 5 min and is washed out for 13 min. (B) Summary of the effects of 0.5 mM CPT-cAMP (percentage inhibition) on TREK-1 current amplitude measured in oocytes at a holding potential of –20 mV and recorded at 22 and 37°C. (C) Voltage ramps of 800 ms duration are applied from a holding potential of –80 mV in a COS cell expressing TREK-1. Currents are measured at 22 and 42°C in the absence and presence of 0.5 mM CPT-cAMP. cAMP is superfused for 2 min. (D) Voltage ramps of 800 ms duration are applied from a holding potential of –80 mV in a COS cell expressing TREK-1. Currents are measured at 22 and 42°C in the absence and presence of 5 µM PGE2 superfused for 2 min. (E) Summary of the experiments performed in COS cells (percentage inhibition) in the presence of 0.5 mM cAMP or 5 µM PGE2 at 22 and 42°C. The mutant Ser333 is not sensitive to both 0.5 mM cAMP and 5 µM PGE2 at 22°C. At 22°C, current amplitude measured at 0 mV is 14.3 ± 3.9 and 98.0 ± 18.5 pA/pF for TREK-1 and TREK-1Ser333 mutant, respectively. At 37°C, current amplitude is 43.7 ± 7.5 and 168.6 ± 28.3 pA/pF for TREK-1 and TREK-1Ser333 mutant, respectively.
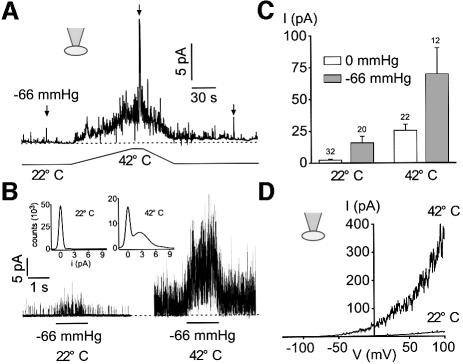
At room temperature, TREK-1 basal activity in the cell-attached patch configuration is negligible and channel activity is increased by stretch (Figure 4A and B). Heat gradually opens TREK-1 at atmospheric pressure, with an increase in channel activity of 17.4 ± 3.8-fold (n = 19) for a 20°C jump (Figure 4A–C). The current induced by heat displays the typical outward rectification and reverses at –80 mV in physiological K+ conditions (Figure 4D). Stretch-induced channel activity (–66 mmHg) is strongly potentiated (9.7 ± 1.9-fold, n = 12) when temperature is increased by 20°C (Figure 4A–C).
Fig. 4. Heat opens TREK-1 in the cell-attached patch configuration. (A) Cell-attached patch recording at 0 mV in a COS cell expressing TREK-1. Channel activity is recorded at atmospheric pressure and during a stretch of –66 mmHg at 22 and 42°C. (B) Channel activity elicited by a stretch of –66 mmHg is largely potentiated at 42°C. The same experiment as in (A). Zero current is indicated by a dotted line. The inset shows Gaussian fits of the amplitude histograms at 22 and 42°C of channel activity recorded at atmospheric pressure (50 bins per histogram). Recordings of 3.5 s duration were filtered at 5 kHz and sampled at 50 kHz. (C) Summary of the experiments (mean current, I) performed at atmospheric pressure and during a stretch of –66 mmHg recorded at 22 and 42°C at 0 mV in cell-attached patches of COS cells expressing TREK-1. (D) Voltage ramps of 800 ms duration from a holding potential of –80 mV in a cell-attached patch from a COS cell expressing TREK-1 recorded at 22 and 42°C.
Heat-induced channel activation is lost upon patch excision (Figure 5). In the outside-out patch configuration, a 20°C increase in temperature fails to affect channel activity, although TREK-1 is strongly opened by AA (Figure 5A). Similarly, in the inside-out patch configuration, heat fails to open TREK-1, while a –66 mmHg stretch, as well as AA (not shown), open channels (Figure 5B). In the inside-out patch configuration, stretch-induced channel activity is not modified significantly by a 20°C increase in temperature (Figure 5B inset; Figure 5C).
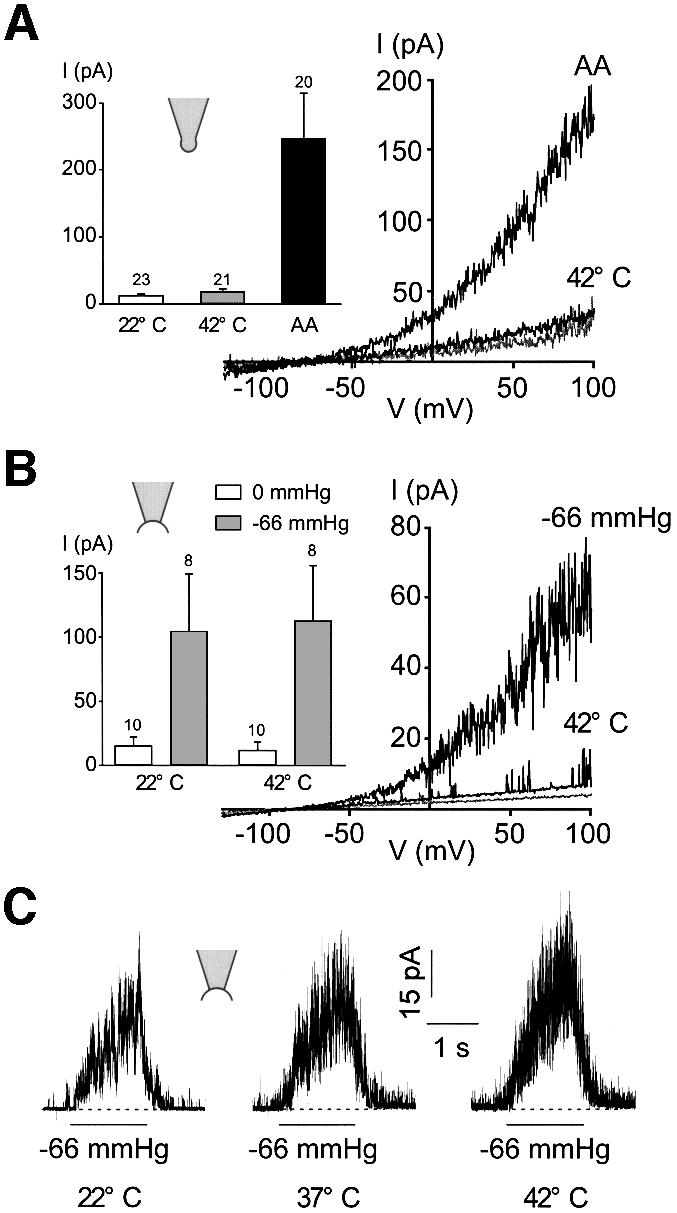
Fig. 5. Heat activation of TREK-1 is lost upon excision. (A) Thermal activation of TREK-1 at 42°C is lost in an outside-out patch, while addition of 10 µM AA at 22°C in the bath solution opens TREK-1 channels. Voltage ramps of 800 ms duration are applied from a holding potential of –80 mV. The inset illustrates the summary of the outside-out patch experiments performed at 22 and 42°C and in the presence of 10 µM AA at 22°C. The patches are held at a holding potential of 0 mV and channel activity is represented as the mean current I. (B) In inside-out patches: heat activation is lost, while a stretch of –66 mmHg opens TREK-1 channels. Voltage ramps of 800 ms duration are applied from a holding potential of –80 mV. The inset illustrates TREK-1 channel activity (mean current I) in inside-out patches measured at 0 mV and recorded at 22 and 42°C at atmospheric pressure and during a stretch of –66 mmHg. (C) A stretch of –66 mmHg elicits TREK-1 opening in an inside-out patch held at 0 mV and is not sensitive to temperature as indicated.
TREK-1 activation by heat is antagonized by an increase in osmolarity (performed with either mannitol or sucrose) (Figure 6A–C). This effect is slightly more pronounced at high temperature (Figure 6B). The inhibition by hyperosmolarity is reversible upon washing out (Figure 6A).
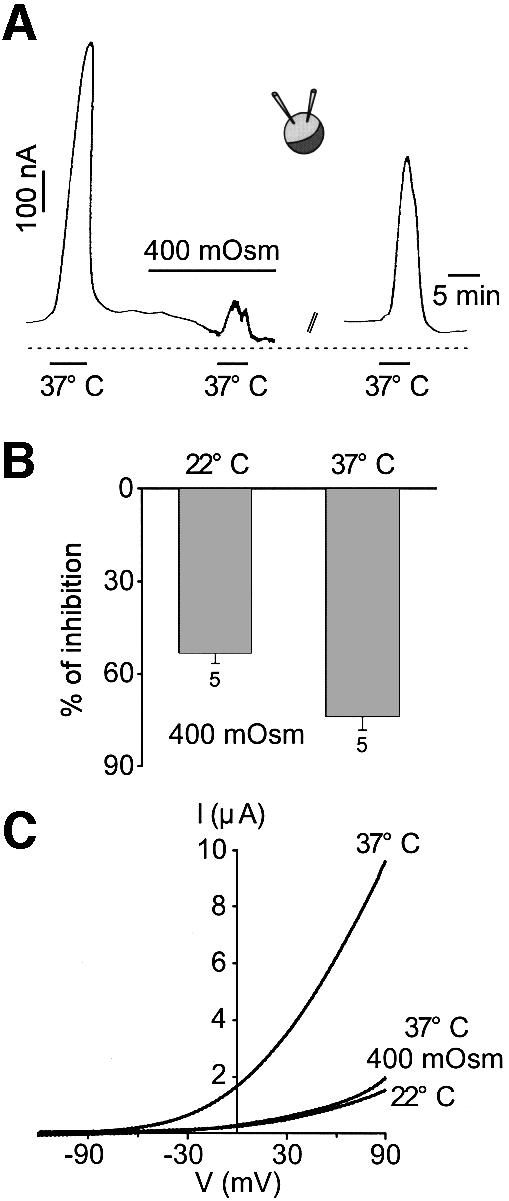
Fig. 6. Hyperosmolarity reverses thermal activation of TREK-1. (A) TREK-1 current is recorded at –20 mV with the two-microelectrode voltage-clamp technique in a Xenopus oocyte. A temperature jump from 22 to 37°C reversibly opens TREK-1. Increasing the osmolarity up to 400 mOsm with mannitol reversibly depresses the opening of TREK-1 by heat. Mannitol is washed out for 20 min. (B) Summary of the experiments illustrating the effect of 400 mOsm hyperosmolarity with mannitol on TREK-1 current amplitude recorded at 22 and 37°C and measured at –20 mV. (C) Voltage ramps of 800 ms duration are applied from a holding potential of –80 mV at 22 and 37°C. A 400 mOsm hyperosmolarity with mannitol reverses TREK-1 current stimulation by heat.
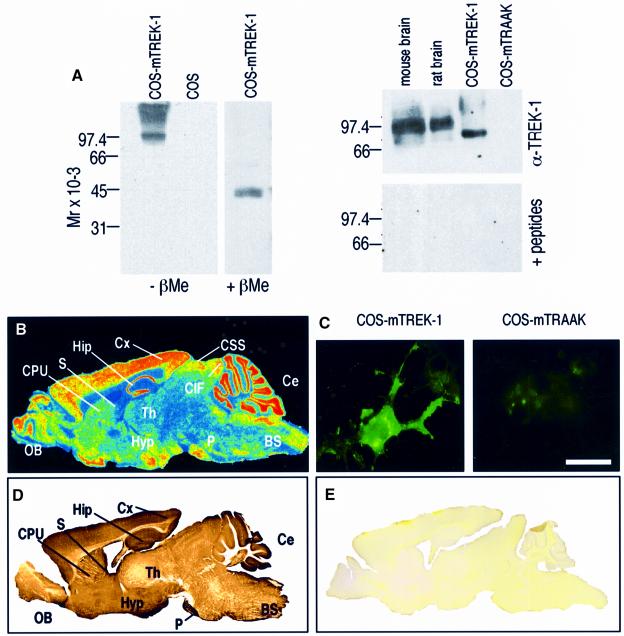
Antibodies directed against TREK-1 were raised in rabbits and affinity purified. On western blots of membrane extracts of TREK-1-transfected COS cells, the antibodies recognize a band with an apparent mol. wt of 84 kDa (Figure 7A, left). No signal is obtained in mock-transfected COS cells under the same experimental conditions. Under reducing conditions in the presence of β-mercaptoethanol (βMe), this band disappears, whereas another one appears that is half the size of the first one and with a molecular weight corresponding to that predicted from the cDNA sequence (45.3 kDa) (Figure 7A, left). These results suggest that TREK-1 forms disulfide-bridged homodimers as previously shown for TWIK-1 (Lesage et al., 1996b), TRAAK (Reyes et al., 2000) and KCNK6 (Salinas et al., 1999). A cysteine residue located in the M1P1 loop of TWIK-1 is implicated in the formation of the interchain disulfide bond. Such a cysteine residue is conserved in the TREK-1 structure (Cys52). On immunoblots of synaptic membranes from mouse or rat brains, the antibodies also recognize a single but more diffuse band corresponding to the dimerized form of TREK-1, but with a size of ∼92–99 kDa, i.e. ∼10 kDa higher than the size observed in transfected COS cells (Figure 7A, right). These differences are probably due to variations in the N-linked glycosylations on residues 95 and 120 located in the extracellular M1P1 loop (Fink et al., 1996) as observed for TWIK-1 and TRAAK (Lesage et al., 1996b; Reyes et al., 2000). When the α-TREK-1 antibodies were pre-incubated with the antigenic fusion peptides before immunoblotting, no signal was observed (Figure 7A, right, lower panel). The specificity of the α-TREK-1 antibodies was also observed in immunocytochemical experiments performed on COS cells transfected with the mouse TREK-1 cDNA (Figure 7C, left panel). No signal was seen on COS cells transfected with mouse TRAAK cDNA (Figure 7C, right panel).
Fig. 7. Characterization of TREK-1 antibodies by western blotting and immunocytochemistry. (A) Left: total proteins of COS cells transfected with mTREK-1 cDNA were separated on a 10% polyacrylamide gel (5 µg/lane) under non-reducing (–βMe) or reducing conditions (+βMe). Western blots were incubated with affinity-purified α-TREK-1 (1:2000) polyclonal rabbit antibodies. Mock-transfected COS cells were used as a negative control (empty expression pCD8 vector). Antigen–antibody complexes were visualized by using an enhanced chemiluminescence method (Super Signal, Pierce). Right: synaptic membranes from mouse and rat brain, and COS cells expressing mTREK-1 or mTRAAK. A 30 µg aliquot of brain synaptic membranes or 5 µg of total proteins of COS cells were loaded per lane. Blots were incubated with α-TREK-1 (1:2000) alone (top) or the α-TREK-1 (1:2000) pre-incubated with a mixture of the two GST fusion proteins (bottom). (B) Digitized autoradiograph illustrating TREK-1 mRNA distribution as observed by in situ hybridization on mouse sagittal brain sections using a specific 49mer oligonucleotide complementary to mouse TREK-1. The same in situ panel has been published previously by Fink et al. (1996) as Figure 3B. The description of the in situ hybridization in this previous work was not correct as it stated that only a very low level of expression of TREK-1 was detected in the hypothalamus. This in situ hybridization shows on the contrary a very strong expression of TREK-1 in the hypothalamic area. (C) Immunocytochemistry experiments on COS cells expressing mTREK-1 (left) or mTRAAK-transfected (right). Immunocomplexes were revealed by fluorescence microscopy. Scale bar, 20 µm. (D) Immunohistochemistry with α-TREK-1 antibody on a sagittal mouse brain section. Immunostaining was visualized using the peroxidase–DAB technique. (E) Signals were blocked by pre-absorption of the α-TREK-1 antibody with an excess of the antigenic fusion proteins. BS, brainstem; Ce, cerebellum; CIF, inferior colliculus; CPU, caudate–putamen; CSS, superior colliculus; Cx, neocortex; Hip, hippocampus; Hyp, hypothalamus; OB, olfactory bulb; P, pontine nuclei; S, septum; Th, thalamus.
Immunohistochemical staining with affinity-purified α-TREK-1 antibodies on sagittal mouse brain slices (Figure 7D) is in excellent agreement with the widespread expression of the TREK-1 mRNA throughout the brain (Figure 7B) (Fink et al., 1996). Signals were blocked by pre-absorption of the α-TREK-1 antibody with an excess of the antigenic fusion proteins (Figure 7E).
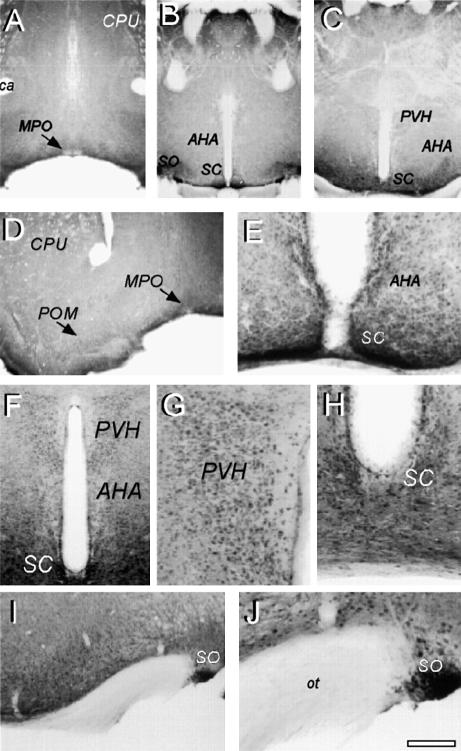
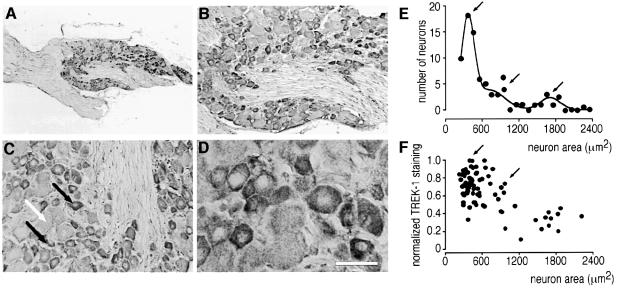
TREK-1 is highly expressed in the rostral hypothalamic regions including the preoptic and anterior hypothalamus, areas known to be implicated in thermoregulation (Figure 8). A pronounced immunostaining was observed in the anterior (Figure 8E and F) and paraventricular (Figure 8F and G) hypothalamus, suprachiasmatic (Figure 8E and H) and supraoptic (Figure 8I and J) nuclei and in the medial and magnocellular preoptic areas (Figure 8D). Intense TREK-1 staining is observed additionally in both small and medium sized sensory neurons of mouse DRG (Figure 9), but is weak in large sensory neurons (Figure 9C–F).
Fig. 8. Immunolocalization of TREK-1 in the mouse preoptic and anterior hypothalamus. Immunohistochemistry was performed on mouse brain coronal sections at the level of PO/AH (A–J). TREK-1 immunostaining was visualized using the peroxidase–DAB technique as described in Materials and methods. (A–C) Low-power microphotographs at brain levels 0.4, –0.7 and –0.9. (D–J) Medium- and high-power microphotographs of distinct areas showing (D) MPO/POM, (E and H) SC, (F and G) PVH and AHA, and (I and J) SO. AHA, anterior hypothalamic area; ca, anterior commissure; CPU, caudate–putamen; MPO, medial preoptic area; ot, optic tract; POM, magnocellular preoptic area; PVH, paraventricular nucleus; SC, suprachiasmatic nucleus; SO, supraoptic nucleus.
Fig. 9. Immunolocalization of TREK-1 in mouse DRG neurons. TREK-1 immunostaining was visualized using the peroxidase–DAB technique as described in Materials and methods. (A–D) TREK-1 immunostaining within DRG at increasing magnification. Note in (C) the high labelling in small and medium sized (black arrows) and low labelling in large (white arrow) sensory neurons. Scale bar, 50 µm. (E) Number of neurons as a function of size (in µm2). The histogram was fitted with the sum of Gaussian functions. Bin size was 50 µm2. (F) Normalized TREK-1 expression as a function of the size of the neurons. Small and medium sized neurons express high levels of TREK-1 (as indicated by arrows).
Discussion
The present report demonstrates that heat gradually and reversibly opens TREK-1 when expressed in Xenopus oocytes and in COS cells. Moreover, TREK-1 is highly expressed in peripheral DRG and central hypothalamic neurons.
Temperature-sensitive free nerve endings are distributed throughout all parts of the skin (Darian-Smith and Johnson, 1977; Darian-Smith, 1984; Spray, 1986). The free nerve endings responsible for temperature detection are of three types. One type, the cold receptor, increases its firing rate as the skin is cooled, with a peak activity at ∼30°C and a further decrease at lower temperatures (Darian-Smith, 1984). The second type of temperature detector, the warm receptor, increases its rate of action potential firing as skin temperature is increased, presents a maximum at 42°C and further activity decreases at higher temperatures (Darian-Smith, 1984). Cold receptors are ∼10–15 times more numerous in any given area of the skin than warm receptors. The third type of temperature detector is a pain receptor that is stimulated by extreme cold or heat (Cesare and McNaughton, 1997; McCleskey, 1997; Reichling and Levine, 1997; Caterina and Julius, 1999; Cesare et al., 1999; Kress and Zeilhofer, 1999). At very cold temperatures (0–12°C), only pain fibres are active. Between 12 and 35°C, cold receptors are stimulated. Nerve fibres from warm receptors are stimulated between 25 and 47°C. Temperatures >47°C not only no longer stimulate warm receptors, but actually stimulate cold receptors (paradoxical cold sensation at noxious high temperature) and pain receptors. The expression of TREK-1 in the small and medium size diameter DRG sensory neurons makes it a possible candidate for temperature sensing. Opening of TREK-1 when warming up to 42°C will polarize cells and contributes to reduced discharge of action potentials. In contrast, cooling will close TREK-1 and thus will lead to cell depolarization and action potential firing. TREK-1 may thus be involved as a temperature sensor in cold fibres that fire action potentials upon cooling. TREK-1 may additionally play an important role in negative feedback regulation of warm-sensitive neurons by tonically limiting action potential firing at warm temperature.
Rostral hypothalamic regions, especially the preoptic and the anterior hypothalamus, are implicated in the regulation of body temperature (Boulant, 1998a,b). Specific hypothalamic neurons are inherently temperature sensitive and retain their sensitivity during synaptic blockade. In most of the spontaneously firing temperature-sensitive hypothalamic neurons, a depolarizing prepotential precedes each action potential. Warming has been shown to speed up the rise of the depolarizing prepotential and consequently to enhance the firing rate of hypothalamic warm-sensitive neurons (Griffin and Boulant, 1995; Griffin et al., 1996). An enhanced inactivation rate of A-type K+ currents at warm temperatures in hypothalamic anterior and preoptic neurons was proposed to be involved in warmth detection (Griffin et al., 1996). Additionally, the different thermosensitivity of resting ionic conductances was proposed to underlie the differential behaviours of warm- and cold-sensitive hypothalamic neurons (Kobayashi and Takahashi, 1993). Expression of TREK-1 in the neurons of the hypothalamus makes it also a possible candidate for cold sensing in the specialized cells as well as a tonic repressor of excitability in warm-sensitive neurons.
Patch excision induces a loss of TREK-1 activation by heat, while stretch still maximally opens channels. This result demonstrates that thermal activation of TREK-1 requires cell integrity and suggests that a cytosolic factor might be involved in channel regulation. A temperature-sensing element functionally attached to TREK-1 might thus regulate its activity. TREK-1 is opened similarly by lipids, including polyunsaturated fatty acids, as well as by intracellular acidosis (Patel et al., 1998; Maingret et al., 1999b, 2000). The C-terminus of TREK-1 is critical for both lipid and acid activations (Patel et al., 1998; Maingret et al., 1999b). Interestingly, in the present report the same region was found to be important for heat activation. The possible role of an intracellular second messenger has been reported previously for a heat-activated cationic conductance in rat sensory neurons (Reichling and Levine, 1997).
PGE2 acting via the EP2 receptor and the second messenger cAMP is a strong sensitizer of sensory neurons to temperature (Cesare and McNaughton, 1997; Cesare et al., 1999; Kress and Zeilhofer, 1999). Chronic pain syndromes involve the sensitization of nociceptors and thermal receptors. Several lines of evidence support the idea that nociceptor responses to temperature are strongly modulated by PKA (Kress and Zeilhofer, 1999). Indeed, the increase in sensitivity to painful stimuli occurs during inflammatory diseases and very often is accompanied by heat hyperalgesia (Kress and Zeilhofer, 1999). Prevention of such sensitization is obviously a potential attractive approach to pain therapy. Similarly, in the hypothalamus, cAMP strongly increases neuronal thermosensitivity by enhancing the thermal response of the prepotential in warm-sensitive neurons (Boulant, 1998b). PGE2 and cAMP have been proposed as prime candidates in the genesis of fever (Philipp-Dormston, 1976; Scammell et al., 1996; Saper, 1998). Aspirin and paracetamol lower PGE2 as well as cAMP levels, and are very efficient in reducing the fever reaction (Philipp-Dormston, 1976; Scammell et al., 1996; Saper, 1998). TREK-1 is inhibited strongly by PGE2 and by cAMP. These effects are mediated via the phosphorylation of Ser333 in the C-terminal region PKA site. It is therefore possible that closing of TREK-1 by the PGE2/cAMP/PKA pathway will lead to cell depolarization and thus might sensitize free nerve endings and modulate fever genesis in central hypothalamic neurons.
In vivo and in vitro evidence suggests a close interaction, in the anterior hypothalamus, between the mechanisms involved in body fluid balance and thermal regulation (Silva and Boulant, 1984; Nakashima et al., 1985; Travis and Johnson, 1993). For instance, increase in plasma osmolarity initiates drinking and raises the thermal set point for sweating, thus reducing evaporative heat loss in dehydrated mammals (Silva and Boulant, 1984; Nakashima et al., 1985; Travis and Johnson, 1993). Inhibition of TREK-1 by hyperosmolarity may thus be involved in such a physiological regulatory mechanism.
Halothane and other inhalational anaesthetics impair normal thermoregulatory processes (Poterack et al., 1991; Farber et al., 1995). Halothane alters the firing rate and thermosensitivity of individual cold- and warm-sensitive neurons in the hypothalamus in the absence of afferent modulation, although it does not affect temperature-insensitive neurons (Poterack et al., 1991; Farber et al., 1995). Alteration of thermosensitive neurons in the preoptic hypothalamic region may result in an imprecision of thermoregulatory responses and represents a potential mechanism by which halothane widens the thermoregulatory threshold range (Farber et al., 1995). Interestingly, TREK-1 is opened by volatile anaesthetics including halothane (Patel et al., 1999). Antidepressant drugs have been reported to alter the circadian pattern of body temperature, and in particular to decrease hypothalamic temperature (Duncan et al., 1995). It was hypothesized that the antidepressant properties of chlorpromazine might be related to its capacity to decrease hypothalamic temperature during sleep (Duncan et al., 1995). Moreover, chlorpromazine is known to alter the stress response to severe cold by reducing the rise in hypothalamic catecholamine and serotonin production (Kortelainen et al., 1989). Chlorpromazine is also a potent blocker of TREK-1 (Patel et al., 1998; Maingret et al., 2000). These pharmacological observations tend to suggest further the important role of TREK-1 in hypothalamic thermoregulative processes.
The presence of lipid-, acid- and stretch-sensitive K+ channels sharing all the properties of TREK-1 has been described previously in hypothalamic neurons as well as in the cell bodies of rat DRG neurons (Kim et al., 1995). Heat activation of these K+ channels may fulfil important physiological functions in these neurons. The maximal heat sensitivity of TREK-1 is observed between 32 and 37°C, with a 0.9-fold increase in current amplitude per degree Centigrade (see Figure 1C and D). Therefore, small physiological changes in temperature, as observed at central thermosensitive neurons, will affect TREK-1 opening maximally. Thermotransduction at peripheral thermosensitive sites occurs at more extreme temperatures, as illustrated by the opening of VR1 and VRL-1 at 43 and 52°C, respectively (Caterina et al., 1997, 1999; Tominaga et al., 1998). The high sensitivity of TREK-1 to thermal stimulation makes this K+ channel a very interesting candidate as a neuronal thermosensor. Since few data are available concerning the ion channels involved in cold transduction, it is difficult to correlate the present findings with previously described cold-sensitive currents in native tissues. It should also be kept in mind that opening of TREK-1 may additionally play a role in negative feedback regulation of warm-sensitive neurons. Further experiments including recording of temperature-sensitive neurons in wild-type as well as in TREK-1 knockout animals will clearly be needed to establish definitively the physiological role of TREK-1 as a temperature sensor.
Materials and methods
COS cell culture, transfection, site-directed mutagenesis, oocyte handling, mRNA injection and electrophysiological procedures have been described extensively elsewhere (Fink et al., 1996, 1998; Lesage et al., 1996a; Duprat et al., 1997; Reyes et al., 1998; Salinas et al., 1999; Maingret et al., 2000). Sylgard-coated patch pipettes with a resistance of 2.5–3 MΩ were used for whole-cell as well as single-channel recordings.
Cell culture
COS-7 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. The 2P domain K+ channel cDNAs were subcloned into the pIRES-CD8 vector and transfected using the DEAE–dextran procedure. Cells were visualized 48 h after transfection using the anti-CD8 antibody-coated beads method.
Solutions
For whole-cell experiments, bath solution (EXT) contained (in mM) 150 NaCl, 5 KCl, 3 MgCl2, 1 CaCl2, 10 HEPES pH 7.4 with NaOH, and pipette solution (INT) contained (in mM) 150 KCl, 3 MgCl2, 5 EGTA and 10 HEPES pH 7.2 with KOH. A K+-rich solution was made by substituting NaCl with KCl. For cell-attached and inside-out patch experiments, the cells were bathed with the INT solution and the pipette contained the EXT solution (5 mM KCl). For oocyte experiments, the bath solution contained (in mM) 96 NaCl, 2 KCl, 2 MgCl2, 1.8 CaCl2, 5 HEPES, 0.5 9-anthracene carboxylic acid pH 7.4 with NaOH (200 mOsm). All chemicals were obtained from Sigma. Stock solutions of AA dissolved in ethanol at the concentration of 100 mM were kept at –20°C and remade weekly. 9-Anthracene carboxylic acid was dissolved in 0.1 M NaOH at a concentration of 100 mM. The temperature of the pre-heated solutions was monitored continuously at the tip of the superfusion apparatus next to the cells under recording. The temperature detector was located 2 mm away from the cell under recording and the flow rate was 0.6 ml/min. The actual temperature at the cell may thus be slightly less than what is measured at the level of the inflow port.
Current and data analysis
Clampex (pclamp) was used to stimulate and store currents. Clampfit (pclamp) and Biopatch (Biologic) were used for current analysis. Mean current amplitude was determined using the Biopatch software. Current traces were filtered at 2 kHz and sampled at 20 kHz for data analysis. Current segments of 1 s duration were analysed to calculate the mean current amplitude. Ratios of current amplitudes under different experimental conditions are given as a fold increase. The data are presented as means associated with their standard errors (SEM).
Mutations
PCR was used to generate N-terminal deletions in TREK-1 by introducing a methionine just before Val47. The C-terminal deletion was generated by introducing a stop codon at Gly308 (Δ103). The C-terminus of TASK-1 was fused to the core of TREK-1 (Val298) to construct a TREK-1–TASK-1 chimeric mutant (TR298/TA248). Ser333 in the consensus PKA phosphorylation site was substituted by alanine.
In situ hybridization
The in situ hybridization procedure has been described previously (Fink et al., 1996).
Preparation of antibodies
Polyclonal anti-rabbit antibodies were prepared as previously described (Lesage et al., 1996b; Salinas et al., 1999; Reyes et al., 2000). DNA fragments encoding the N-terminal part (from Met1 to Trp44) and the M1P1 domain (Pro71–Ile114) of TREK-1 were amplified by PCR and subcloned in-frame downstream of the glutathione S-transferase (GST) sequence into the pGEX3X plasmid (Pharmacia). The corresponding fusion proteins were produced in Escherichia coli and purified on glutathione–Sepharose according to the manufacturer’s instructions (Pharmacia). Antibodies directed against a mixture of the two fusion proteins were raised in New Zealand rabbits. For the purification of anti-TREK-1 (α-TREK-1) antibodies, the antibodies directed against the GST were first depleted from the immune serum by absorption on nitrocellulose strips saturated with GST. The α-TREK-1 antibodies were then purified by affinity against nitrocellulose strips saturated with the GST–TREK-1 fusion proteins as previously described for TRAAK antibodies (Reyes et al., 2000).
COS cell immunofluorescence
The procedure was carried out as previously described (Lesage et al., 1996b; Salinas et al., 1999; Reyes et al., 2000). Briefly, TREK-1-transfected cells on coverslips were fixed in phosphate-buffered 4% paraformaldehyde (PFA/PBS), blocked in goat serum diluted 1:20 in PBS, 2% bovine serum albumin, 0.1% Triton X-100 and incubated with α-TREK-1 (1:500) followed by an incubation with fluorescein isothiocyanate (FITC)-coupled anti-rabbit IgGs (1:500; Jackson Laboratories). The immunocomplexes were visualized by fluorescence microscopy.
Immunoblot analysis
For western blot analysis, 5 µg of TREK-1-transfected COS cell proteins, mock-transfected COS cells or 30 µg of synaptic membrane proteins from mouse or rat brain were resolved by SDS–PAGE and transferred onto nitrocellulose membranes (Hybond C-extra; Amersham). Blots were blocked with PBS, 0.1% Tween-20, 4% low-fat dry milk and then incubated with affinity-purified α-TREK-1 (1:1000–1:2000). Blots were then incubated with peroxidase-conjugated goat anti-rabbit or anti-mouse IgGs (1:20 000; Jackson) and the immunocomplexes were revealed by using an enhanced chemiluminescence method (Super Signal, Pierce).
Preparation of tissue sections and immunohistochemistry
BalbC mice were killed after transcardial perfusion with a 4% PFA/PBS solution. Dissected tissues were post-fixed in the same solution for 2 h, cut on a vibratome (Leica) (50 µm) and stored in a non-freezing phosphate-buffered glycerol/ethylene glycol solution at –20°C until use. Frozen paraformaldehyde-fixed mouse DRG sections (10 µm) were cut on a cryostat (Leica). Sections were washed and permeabilized in 0.1% Triton X-100 for 1 h for brain sections and 20 min for DRG sections. Endogenous peroxidase was then inhibited in 3% H2O2 in PBS for 30 min. After washing, sections were blocked in 3% goat serum for at least 3 h. Floating sections were then incubated with TREK-1 antibodies (1:250–1:500) in 1% goat serum at 4°C with constant rotary shaking for ∼24–36 h. After washing (six changes/60 min total), labelling was developed according to the Vectastain Elite ABC kit instructions (Vector Labs). The antigen–antibody complexes were visualized by reaction with the chromogen 3,3′-diaminobenzidine-tetrahydrochloride (DAB) and H2O2 with or without nickel amplification. In control procedures, anti-TREK-1 antibodies were either omitted or pre-absorbed with the GST fusion proteins before being added to the tissue sections. TREK-1 immunostaining was analysed with the NIH image software allowing measurements of light density as well as neuron area.
Acknowledgments
Acknowledgements
We wish to thank M.Jodar and V.Lopez for excellent technical assistance. This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Association pour la Recherche sur le Cancer (ARC), the Conseil Regional (PACA) and the Association Française contre les Myopathies (AFM).
References
- Boulant J.A. (1998a) Cellular mechanisms of temperature sensitivity in hypothalamic neurons. Prog. Brain Res., 115, 3–8. [DOI] [PubMed] [Google Scholar]
- Boulant J.A. (1998b) Hypothalamic neurons. Mechanisms of sensitivity to temperature. Ann. N Y Acad. Sci., 856, 108–115. [DOI] [PubMed] [Google Scholar]
- Braun H.A., Bade,H. and Hensel,H. (1980) Static and dynamic discharge patterns of bursting cold fibers related to hypothetical receptor mechanisms. Pflugers Arch., 386, 1–9. [DOI] [PubMed] [Google Scholar]
- Buckler K.J. (1997) A novel oxygen-sensitive potassium current in rat carotid body type I cells. J. Physiol., 498, 649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M.J. and Julius,D. (1999) Sense and specificity: a molecular identity for nociceptors. Curr. Opin. Neurobiol., 9, 525–530. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Schumacher,M.A., Tominaga,M., Rosen,T.A., Levine,J.D. and Julius,D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature, 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Rosen,T.A., Tominaga,M., Brake,A.J. and Julius,D. (1999) A capsaicin-receptor homologue with a high threshold for noxious heat. Nature, 398, 436–441. [DOI] [PubMed] [Google Scholar]
- Cesare P. and McNaughton,P. (1997) Peripheral pain mechanisms. Curr. Opin. Neurobiol., 7, 493–499. [DOI] [PubMed] [Google Scholar]
- Cesare P., Moriondo,A., Vellani,V. and McNaughton,P.A. (1999) Ion channels gated by heat. Proc. Natl Acad. Sci. USA, 96, 7658–7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I. (1984) Thermal sensibility. In Handbook of Physiology, The Nervous System. Vol. III, American Physiological Society, Bethesda, MA, pp. 879–913. [Google Scholar]
- Darian-Smith I. and Johnson,K.O. (1977) Thermal sensibility and thermoreceptors. J. Invest. Dermatol., 69, 146–153. [DOI] [PubMed] [Google Scholar]
- Duncan W.C., Johnson,K.A. and Wehr,T.A. (1995) Antidepressant drug-induced hypothalamic cooling in Syrian hamsters. Neuropsychopharmacology, 1, 17–37. [DOI] [PubMed] [Google Scholar]
- Duprat F., Lesage,F., Fink,M., Reyes,R., Heurteaux,C. and Lazdunski,M. (1997) TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J., 16, 5464–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber N.E., Schmidt,J.E., Kampine,J.P. and Schmeling,W.T. (1995) Halothane modulates thermosensitive hypothalamic neurons in rat brain slices. Anesthesiology, 83, 1241–1253. [DOI] [PubMed] [Google Scholar]
- Fink M., Duprat,F., Lesage,F., Reyes,R., Romey,G., Heurteaux,C. and Lazdunski,M. (1996) Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J., 15, 6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M., Lesage,F., Duprat,F., Heurteaux,C., Reyes,R., Fosset,M. and Lazdunski,M. (1998) A neuronal two P domain K+ channel activated by arachidonic acid polyunsaturated fatty acid. EMBO J., 17, 3297–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J.D. and Boulant,J.A. (1995) Temperature effects on membrane potential and input resistance in rat hypothalamic neurones. J. Physiol., 488, 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J.D., Kaple,M.L., Chow,A.R. and Boulant,J.A. (1996) Cellular mechanisms for neuronal thermosensitivity in the rat hypothalamus. J. Physiol., 492, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Sladek,C.D., Aguadovelasco,C. and Mathiasen,J.R. (1995) Arachidonic acid activation of a new family of K+ channels in cultured rat neuronal cells. J. Physiol., 484, 643–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S. and Takahashi,T. (1993) Whole-cell properties of temperature-sensitive neurons in rat hypothalamic slices. Proc. R. Soc. Lond. B Biol. Sci., 251, 89–94. [DOI] [PubMed] [Google Scholar]
- Kortelainen M.L., Lapinlampi,T. and Hirvonen,J. (1989) Chlorpromazine-induced alterations in hypothalamic amine metabolism and stress responses in severe cold. Z. Rechtsmed., 102, 377–390. [DOI] [PubMed] [Google Scholar]
- Kress M. and Zeilhofer,H.U. (1999) Capsaicin, protons and heat: new excitement about nociceptors. Trends Pharmacol. Sci., 20, 112–118. [DOI] [PubMed] [Google Scholar]
- Lauritzen I., Blondeau,N., Heurteaux,C., Widmann,C., Romey,G. and Lazdunski,M. (2000) Polyunsaturated fatty acids are potent neuroprotectors. EMBO J., 19, 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F., Guillemare,E., Fink,M., Duprat,F., Lazdunski,M., Romey,G. and Barhanin,J. (1996a) TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J., 15, 1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Lesage F., Reyes,R., Fink,M., Duprat,F., Guillemare,E. and Lazdunski,M. (1996b) Dimerization of TWIK-1 K+ channel subunits via a disulfide bridge. EMBO J., 15, 6400–6407. [PMC free article] [PubMed] [Google Scholar]
- Maingret F., Fosset,M., Lesage,F., Lazdunski,M. and Honoré,E. (1999a) TRAAK is a neuronal mechano-gated K+ channel. J. Biol. Chem., 274, 1381–1387. [DOI] [PubMed] [Google Scholar]
- Maingret F., Patel,A.J., Lesage,F., Lazdunski,M. and Honoré,E. (1999b) Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J. Biol. Chem., 274, 26691–26696. [DOI] [PubMed] [Google Scholar]
- Maingret F., Patel,A., Lesage,F., Lazdunski,M. and Honoré,E. (2000) Lysophospholipids open the two P domain mechano-gated K+ channels TREK-1 and TRAAK. J. Biol. Chem., 275, 10128–10133. [DOI] [PubMed] [Google Scholar]
- McCleskey E.W. (1997) Thermoreceptors: recent heat in thermosensation. Curr. Biol., 7, R679–R681. [DOI] [PubMed] [Google Scholar]
- Nagy I. and Rang,H. (1999a) Noxious heat activates all capsaicin-sensitive and also a sub-population of capsaicin-insensitive dorsal root ganglion neurons. Neuroscience, 88, 995–997. [DOI] [PubMed] [Google Scholar]
- Nagy I. and Rang,H.P. (1999b) Similarities and differences between the responses of rat sensory neurons to noxious heat and capsaicin. J. Neurosci., 19, 10647–10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T., Hori,T., Kiyohara,T. and Shibata,M. (1985) Osmosensitivity of preoptic thermosensitive neurons in hypothalamic slices in vitro. Pflugers Arch., 405, 112–117. [DOI] [PubMed] [Google Scholar]
- Patel A.J., Honoré,E., Maingret,F., Lesage,F., Fink,M., Duprat,F. and Lazdunski,M. (1998) A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J., 17, 4283–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.J., Honoré,E., Lesage,F., Fink,M., Romey,G. and Lazdunski,M. (1999) Inhalational anaesthetics activate two-pore-domain background K+ channels. Nature Neurosci., 2, 422–426. [DOI] [PubMed] [Google Scholar]
- Philipp-Dormston W.K. (1976) Evidence for the involvement of adenosine 3′,5′-cyclic monophosphate in fever genesis. Pflugers Arch., 362, 223–227. [DOI] [PubMed] [Google Scholar]
- Poterack K.A., Kampine,J.P. and Schmeling,W.T. (1991) The effect of halothane on thermosensitive neurons in the preoptic region of the anterior hypothalamus in acutely instrumented cats. Anesthesiology, 75, 625–633. [DOI] [PubMed] [Google Scholar]
- Reichling D.B. and Levine,J.D. (1997) Heat transduction in rat sensory neurons by calcium-dependent activation of a cation channel. Proc. Natl Acad. Sci. USA, 94, 7006–7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes R., Duprat,F., Lesage,F., Fink,M., Farman,N. and Lazdunski,M. (1998) Cloning and expression of a novel pH-sensitive two pore domain potassium channel from human kidney. J. Biol. Chem., 273, 30863–30869. [DOI] [PubMed] [Google Scholar]
- Reyes R., Lauritzen,I., Lesage,F., Ettaiche,M., Fosset,M. and Lazdunski,M. (2000) Immunolocalization of the arachidonic-acid and mechano-sensitive baseline TRAAK potassium channel in the nervous system. Neuroscience, 893–901. [DOI] [PubMed] [Google Scholar]
- Salinas M., Reyes,R., Lesage,F., Fosset,M., Heurteaux,C., Romey,G. and Lazdunski,M. (1999) Cloning of a new mouse two-P domain channel subunit and a human homologue with a unique pore structure. J. Biol. Chem., 274, 11751–11760. [DOI] [PubMed] [Google Scholar]
- Saper C.B. (1998) Neurobiological basis of fever. Ann. N Y Acad. Sci., 856, 90–94. [DOI] [PubMed] [Google Scholar]
- Scammell T.E., Elmquist,J.K., Griffin,J.D. and Saper,C.B. (1996) Ventromedial preoptic prostaglandin E2 activates fever-producing autonomic pathways. J. Neurosci., 16, 6246–6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer K. and Braun,H.A. (1992) Modulation of cutaneous cold receptor function by electrolytes, hormones and thermal adaptation. Physiol. Res., 41, 71–75. [PubMed] [Google Scholar]
- Silva N.L. and Boulant,J.A. (1984) Effects of osmotic pressure, glucose and temperature on neurons in preoptic tissue slices. Am. J. Physiol., 247, R335–R345. [DOI] [PubMed] [Google Scholar]
- Spray D.C. (1986) Cutaneous temperature receptors. Annu. Rev. Physiol., 48, 625–638. [DOI] [PubMed] [Google Scholar]
- Tominaga M., Caterina,M.J., Malmberg,A.B., Rosen,T.A., Gilbert,H., Skinner,K., Raumann,B.E., Basbaum,A.I. and Julius,D. (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron, 21, 531–543. [DOI] [PubMed] [Google Scholar]
- Travis K.A. and Johnson,A.K. (1993) In vitro sensitivity of median preoptic neurons to angiotensin II, osmotic pressure and temperature. Am. J. Physiol., 264, R1200–R1205. [DOI] [PubMed] [Google Scholar]