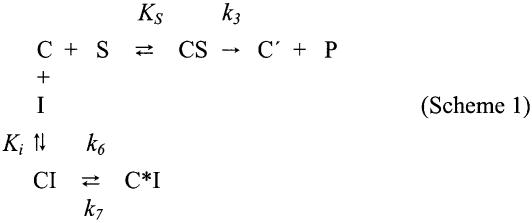Abstract
Chloramphenicol is thought to interfere competitively with the binding of the aminoacyl-tRNA 3′-terminus to ribosomal A-site. However, noncompetitive or mixed-noncompetitive inhibition, often observed to be dependent on chloramphenicol concentration and ionic conditions, leaves some doubt about the precise mode of action. Here, we examine further the inhibition effect of chloramphenicol, using a model system derived from Escherichia coli in which a peptide bond is formed between puromycin and AcPhe-tRNA bound at the P-site of poly(U)-programmed ribosomes, under ionic conditions (6 mM Mg2+, 100 mM NH4+, 100 µM spermine) more closely resembling the physiological status. Kinetics reveal that chloramphenicol (I) reacts rapidly with AcPhe-tRNA·poly(U)·70S ribosomal complex (C) to form the encounter complex CI which is then isomerized slowly to a more tight complex, C*I. A similar inhibition pattern is observed, if complex C modified by a photoreactive analogue of spermine, reacts in buffer free of spermine. Spermine, either reversibly interacting with or covalently attached to ribosomes, enhances the peptidyltransferase activity and increases the chloramphenicol potency, without affecting the isomerization step. As indicated by photoaffinity labeling, the peptidyltransferase center at which chloramphenicol binds, is one of the preferred cross-linking sites for polyamines. This fact may explain the effect of spermine on chloramphenicol binding to ribosomes.
INTRODUCTION
Chloramphenicol (CAM), despite its limited use in developed countries following concerns about toxicity, is still the drug of choice for topical treatment of a wide variety of bacterial infections including serious anaerobic infections (1). CAM binds to the 50S ribosomal subunit and blocks fundamental ribosomal functions, such as peptidyltransferase (PTase) activity (2–7), binding and movement of ribosomal substrates through the PTase center (8–10) and translation termination (11–13). Recently, it has been reported that CAM also causes translational inaccuracy in vivo (14).
CAM and puromycin have been considered as iso-structural both with one another and with the 3′-end of aminoacyl-tRNA (10). Various binding studies reviewed by Pongs (15) and recent crystallographic studies in ribosome–CAM complexes (16) reinforce this consideration. Consistently, CAM has been found to inhibit competitively the reaction between puromycin and P-site-bound AcPhe-tRNA (2,6). In contrast to these observations, the drug surprisingly affects neither the puromycin reaction with Ac(Phe)2-tRNA, nor the transpeptidation of the AcPhe-residue to Phe-tRNA; nevertheless, it enhances the release of oligo(Phe)-tRNAs from the P-site (9). Moreover, at least 11 bases in the central loop of domain V are implicated in CAM binding, as detected by chemical probing (17,18) and mutational analysis of 23S rRNA (19). Many of these positions are also protected by bound tRNA at the A- or P-site, or by antibiotics either interfering with peptide bond formation or blocking peptide elongation by steric hindrance with the growing polypeptide chain. Such a complicated situation is consistent with the suggestion that CAM bound to ribosome may overlap or influence by allosteric effects both the binding site of the terminal –CCA of aminoacyl-tRNA at the A-site and the entry site of the peptidyl channel (9).
In the course of kinetic studies, the problem of whether CAM behaves as a simple competitive inhibitor of peptide bond formation remains unresolved. Besides competitive kinetics, there is also evidence for noncompetitive (20) or mixed-noncompetitive modes of action (3,5,6), depending on the inhibitor concentration and the buffer system used. Experiments from more than 30 years ago have demonstrated that both monovalent and Mg2+ ions at optimal concentrations (21,22) are essential components for the PTase activity and the interaction of CAM with ribosomes. The rationale for the interdependence of monovalent/Mg2+ ions and CAM interaction with ribosomes is provided in a recent publication of the high-resolution crystal structure of Haloarcula marismortui 50S ribosomal subunit (23); a K+ ion is situated in close proximity to nucleoside A2451 and in coordination to G2061 and G2447 (Escherichia coli numbering). As proposed by Bayfield et al. (24), this ion is dissociated in inactive ribosomes, and this dissociation might also account for the inability of inactive ribosomes to bind CAM. In another crystallographic study (16), two hydrated ions of Mg2+ have been found in crystals of Deinococcus radiodurans 50S subunits complexed with CAM, overlapping with the CAM binding site and mediating the interaction of CAM groups with nucleosides of the catalytic center. Nevertheless, in addition to monovalent and divalent ions, polyamines are also essential for establishing a protein-synthesizing in vitro system with near in vivo characteristics (25). It should be mentioned that spermine has been implicated in the interaction of ribosomes with erythromycin (26), an antibiotic sharing a set of common binding features with CAM.
Puromycin binding to ribosomes already bearing an active donor substrate, results in peptide bond formation (27). This model reaction has been used almost exclusively in the kinetic studies of inhibition of peptide bond formation by CAM. Most of these studies utilizing conventional buffers, have been analyzed on the assumption that the interaction between ribosome (R) and CAM (I) can be expressed by a fast equilibrium of the form, R + I ⇄ RI. In this study, we re-examine the CAM problem with a somewhat different rationale. First, since polyamines are strictly required for efficient in vitro and in vivo protein synthesis, we use a polyamine buffer (6 mM Mg2+, 100 mM NH4+, 100 µM spermine), instead of conventional buffers. Second, we analyze the puromycin reaction as a pseudo-first-order reaction and thus we have a complete picture of the entire course of the reaction (progress curve). Using this system, we indicate that CAM behaves as a competitive slow-binding inhibitor, interacting with the ribosomal complex via a two-step mechanism. This may explain the discrepancy between the various reports on the kinetics of inhibition of peptide-bond formation by CAM. Furthermore, this system allows us to reveal kinetically the contribution of spermine in the interaction of CAM with ribosomes. Our kinetic results correlate well with cross-linking data, implying that spermine bound at the vicinity of the CAM binding pocket influences the interaction of CAM with ribosomes, either directly or by altering the local conformation of ribosomes.
MATERIALS AND METHODS
Materials
Puromycin dihydrochloride (disodium salt), chloramphenicol, spermine tetrahydrochloride, dimethyl sulfate (DMS), DMS stop solution and heterogeneous tRNA from E.coli were supplied by Sigma (St Louis, MO, USA). l-[2,3,4,5,6-3H]Phenylalanine and [14C]spermine were purchased from Amersham Biosciences Inc. (Piscataway, NJ, USA). AMV reverse transcriptase and RNase H were from Roche Diagnostics (Mannheim, Germany) and Promega (Madison, WI, USA), respectively. dNTPs and ddNTPs were obtained from Boehringer (Mannheim, Germany). Cellulose nitrate filters (type HA; 24-mm diameter, 0.45-µm pore size) were from Millipore Corporation (Bedford, MA, USA). N1-azidobenzamidino (ABA)-spermine was synthesized from methyl 4-azido benzoimidate and spermine, and purified by chromatography on a sulfopropyl-Sephadex column (28).
Biochemical preparations
Salt-washed (0.5 M NH4Cl) 70S ribosomes and partially purified translation factors were obtained from E.coli CAN20–12E cells as reported previously (29). 23S rRNA was isolated from 50S ribosomal subunits by phenol extraction and purified by electrophoresis on 8% polyacrylamide/7 M urea gels. Initiation ribosomal complex, i.e. the Ac[3H]Phe-tRNA· poly(U)·70S ribosome complex (complex C), was enzymatically prepared at 6 mM Mg2+ and 100 mM NH4+ in the presence or in the absence of 100 µM spermine, and purified through absorption on cellulose nitrate filters as described previously (30). Approximately 20% of the absorbed ribosomes were in the form of complex C. More than 95% of this complex was reactive toward puromycin, implying that the bound Ac[3H]Phe-tRNA was almost exclusively positioned at the P-site.
Photo-affinity labeling, chemical modification and mapping of cross-linking sites of ABA-spermine in 23S rRNA
Complex C was photolabeled with 100 µM ABA-[14C]spermine as reported previously (31). After the irradiation step, the incubation mixture was treated with 100 µM dithiothreitol and separated from non-covalently bound photoprobe by gel filtration on Sephadex G50 and micro-dialysis (32). Aliquots of labeled or non-labeled complex C (100 pmol) were modified with DMS as reported (32). Approximate localization of ABA-spermine cross-linking sites in 23S rRNA was done by hybridization of complex C with selected pairs of 11-deoxynucleotides complementary to 23S rRNA at positions 200 nt apart, followed by digestion with RNase H and analysis of digestion products by gel electrophoresis (32). Precise identification of the cross-linking sites was made by primer extension analysis (32). In each of the experiments, control samples were run in parallel with unmodified ribosomes or ribosomes photolabeled in the simultaneous presence of a 250-fold excess of spermine.
Peptide bond formation assay and first-order analysis
The reaction between photolabeled or untreated complex C and excess puromycin (S) was performed at 25°C in the presence of 6 mM Mg2+ and 100 mM NH4+. When required, 100 µM spermine was also included in the reaction mixture. Under these conditions, the puromycin reaction
displayed pseudo-first-order kinetics and was analyzed as described previously (30). At each concentration of puromycin, the first-order rate constant kobs was determined by fitting the x values into equation 1
where x is the percent of Ac[3H]Phe-tRNA bound at the P-site of complex C that is converted to product (P), and t is the reaction time. It should be mentioned that the x values were corrected, taking into account the parallel inactivation of complex C during the puromycin reaction and the intervention of other species, except for complex C. kobs was related to the puromycin concentration by the relationship
and from the double-reciprocal plot of equation 2 the values of k3 and KS could be estimated by linear regression (Microcal Origin, Version: 6.00, provided by Microcal Software, Inc.).
In the presence of CAM, biphasic logarithmic time plots were obtained. The slope of the straight line through the origin was called the initial slope and was taken as the value of the apparent rate constant, (kobs)o, at the early phase of the reaction. Similarly, the slope of the second straight line was taken as the value of the apparent constant, (kobs)s, at the late phase of the puromycin reaction. All data presented in the figures denote the mean values obtained from four independent experiments. To estimate the data variability, one-way analysis of variance (ANOVA) procedure was used. Whenever differences between mean values were found, the F-Scheffé test was used to determine which means were significantly different from each other. All statistical tests were made using an SPSS program for MS Windows, Release 6.0.
RESULTS
Inhibition of peptide bond formation by CAM
The reaction between complex C and excess puromycin carried out at 25°C, displays pseudo-first-order kinetics. It proceeds as an irreversible reaction in which C is converted to an inactive complex, C′. Since in the presence of polyamines, both optimal binding of AcPhe-tRNA to ribosomes and optimal PTase activity require 6 mM Mg2+ and 100 mM NH4+ (6,33), all subsequent experiments are performed under these ionic conditions. Equation 1 predicts that the progress curve of the puromycin reaction is a straight line. Such a plot obtained at 400 µM puromycin is given in Figure 1 (upper line). However, when AcPhe-puromycin synthesis is carried out in the presence of CAM, an early as well as a late phase can be seen clearly in the progress curves (Fig. 1, four lower lines). The deviation from linearity observed in the presence of CAM is of vital importance for the present analysis, since it reveals a delay in the onset of inhibition. This finding combined with the fact that the initial slope of progress curves varies as a function of the inhibitor concentration, suggests that CAM (I) reacts rapidly with complex C to form the encounter complex CI, which is then isomerized slowly to a tighter complex, termed C*I. Consequently, a kinetic model which could adequately explain the above-mentioned results is that shown in Scheme 1
Figure 1.
First-order time plots for the AcPhe-puromycin synthesis in the presence or absence of chloramphenicol. Complex C reacted at 25°C in the presence of 6 mM Mg2+ and 100 mM NH4+, with (open circles) 400 µM puromycin or with a solution containing 400 µM puromycin and chloramphenicol at (filled circles) 3 µM, (open squares) 6 µM, (filled squares) 12 µM and (diamonds) 18 µM. The deviation from linearity observed in the presence of chloramphenicol reveals a delay on the onset of inhibition.
Scheme 1.
According to Scheme 1, kinetics (see Appendix) predict that the inhibition at both phases, early and late, is of the competitive type. In Figure 2, we show the double-reciprocal plots of the early (kobs)o and late slopes (kobs)s, obtained at several concentrations of puromycin. The type of inhibition in both cases is simple competitive, as evidenced by the corresponding slope replots shown in Figure 3. Both replots are linear and meet the vertical axis at the same point. This point coincides with the slope obtained from the corresponding control experiment ([CAM] = 0), a fact supporting the mechanism given by Scheme 1. Each straight line shown in Figure 3 when extrapolated meets the horizontal axis at a point pertaining to the inhibition-constant, which is for the early and late phase of reaction equal to 3.1 and 0.88 µM, respectively. Once both Ki and Ki* are calculated, the isomerization constant k6/k7 can be determined by equation A14 (see Appendix). The value of this constant equals 2.52.
Figure 2.
Double-reciprocal plots for the AcPhe-puromycin synthesis in the presence or absence of chloramphenicol. The data presented in panels A and B were collected from the early (t < 15 s) and the late phases (t > 1 min) of logarithmic time plots, respectively, such as those shown in Figure 1. The reaction was carried out at 6 mM Mg2+ and 100 mM NH4+, in the absence (open circles) or in the presence of chloramphenicol at (filled circles) 3 µM, (open squares) 6 µM, (filled squares) 12 µM and (diamonds) 18 µM. The inhibition at both phases of the reaction, early and late, is of the competitive type.
Figure 3.
Slope replots (slopes of double-reciprocal plots versus chloramphenicol concentration). The data were obtained from the double-reciprocal plots of (open circles) Figure 2A and (filled circles) 2B. The linearity of slope replots confirms the competitive character of inhibition and indicates that only one inhibitor binding site is implicated in both phases of the reaction.
Adopting the slow-onset inhibition theory in our study, the apparent equilibration rate constant, k′, for the attainment of equilibrium between complex C and CAM can be estimated from the intersection point of the two linear parts of progress curve; at this point, k′ = 1/t (34). For instance, the progress curve at 400 µM puromycin and 18 µM CAM gives a k′ value equal to 2.8 min–1 (Fig. 1). To calculate the individual values of k6 and k7, the k′ values at each concentration of puromycin are first replotted versus [CAM] (Fig. 4). These plots are hyperbolic, in agreement with the two-step inhibition mechanism. From these plots and equation A8, provided that it holds, we find by non-linear regression that k6 and k7 are equal to 2.29 and 0.99 min–1, respectively. k6 and k7 values measured at different concentrations of puromycin, do not vary by more than 6%.
Figure 4.
Variation of the apparent equilibration rate constant, k′, as a function of chloramphenicol concentration. The reaction was carried out at 6 mM Mg2+ and 100 mM NH4+ in the presence of puromycin at (filled circles) 2 mM, (open circles) 400 µM, (triangles) 200 µM and (squares) 100 µM. The k′ value at each concentration of puromycin and chloramphenicol was estimated from the intersection point of the two linear parts of the corresponding progress curve (for example, see lower line in Fig. 1). The hyperbolic character of plots reveals that chloramphenicol inhibits the puromycin reaction by a two-step mechanism.
Spermine reversibly interacting with or covalently attached to ribosomes, renders the inhibitory effect of CAM stronger at 6 mM Mg2+
We have previously observed at 6 mM Mg2+ and 100 mM NH4+ that addition of spermine at concentrations up to 150 µM improves the activity of PTase (30). On the other hand, the early report by Vogel et al. (22) provides evidence for a correlation between PTase activity and the ability of ribosomes to interact with CAM. For this reason, we have re-examined the mechanism of inhibition using complex C that is prepared and interacts with puromycin in the presence of 100 µM spermine. Such a complex possesses 50% higher activity, compared with that prepared in the absence of polyamines (Table 1). Despite its stimulatory effect on PTase activity, spermine does not change the type of CAM inhibition. Thus, CAM inhibits peptide-bond formation by binding initially to complex C in competition with puromycin. Subsequently, as a result of conformational changes a slow isomerization, CI ⇄ C*I, occurs. However, the values of certain kinetic parameters differ from those obtained in the absence of spermine (Table 1). Namely, the Ki value is reduced from 3.1 to 0.7 µM, whereas the isomerization constants k6 and k7 remain essentially unchanged. As a consequence, the dissociation constant Ki* concerning both steps of CAM interaction with complex C is decreased from 0.88 to 0.2 µM. Complex C photolabeled with ABA-spermine exhibits similar affinity for CAM. Both Ki and Ki* values are 4-fold lower than those obtained in the absence of polyamines, while the isomerization step is not affected.
Table 1. Equilibrium and kinetic constants derived from analysis of the inhibition of AcPhe-puromycin synthesis by chloramphenicola.
| Puromycin reaction at: | |||
|---|---|---|---|
| Constant | 6 mM Mg2+, 100 mM NH4+ | 6 mM Mg2+, 100 mM NH4+, 100 µM spermine | 6 mM Mg2+, 100 mM NH4+ with complex C photolabeled by 100 µM ABA-spermine |
| k3 (min–1) | 2.00 ± 0.04 | 2.86 ± 0.05 | 2.60 ± 0.05 |
| Ks (µM) | 625 ± 31 | 623 ± 28 | 621 ± 25 |
| Ki (µM) | 3.10 ± 0.30 | 0.70 ± 0.15 | 0.80 ± 0.07 |
| Ki* (µM) | 0.88 ± 0.10 | 0.20 ± 0.04 | 0.24 ± 0.02 |
| k6/k7 | 2.52 ± 0.16 | 2.50 ± 0.13 | 2.33 ± 0.15 |
| k6 (min–1) | 2.29 ± 0.13 | 2.24 ± 0.07 | 2.34 ± 0.07 |
| k7 (min–1) | 0.99 ± 0.04 | 0.95 ± 0.04 | 1.06 ± 0.07 |
aData represent the mean ± S.E. values obtained from four independently performed experiments.
ABA-spermine cross-linking sites in 23S rRNA are localized adjacently to CAM binding site
A prerequisite for interpretation of the spermine effect on the mechanism of CAM action is the elucidation of CAM and polyamine interactions with ribosomal constituents. Despite the fact that much has been learnt about the CAM interactions with ribosome either in its crystalline conformation or in solution, there is still relatively little information available concerning the contacts between polyamines and the large ribosomal subunit. To gain a comprehensive view of the 23S rRNA nucleotide residues involved in polyamine binding, we have used a photoreactive analogue of spermine, ABA-spermine, that has an arylazido group attached to one of the terminal amino groups of the molecule. Under irradiation at 300 nm, the arylazido group of ABA-spermine is converted to nitrene, a highly reactive group that is able to form covalent bonds with a variety of adjacent groups of the target molecule (28). Results obtained from analysis of ABA-[14C]spermine cross-linking to domain V of 23S rRNA by RNase H digestion, are shown in Figure 5. Interestingly, the region encompassing the PTase loop appears to be susceptible to ABA-spermine cross-linking. Experiments run in the presence of a 250-fold excess of spermine, show that the radioactive labeling of 23S rRNA is essentially abolished under these conditions (Fig. 5, lane 2).
Figure 5.

Analysis of ABA-spermine cross-links in 23S rRNA by RNase H digestion. 23S rRNA from complex C photolabeled with 100 µM ABA-[14C]spermine, was incubated with cDNA probes, and then digested with RNase H. The products of digestion were resolved on 5% polyacrylamide/ 7 M urea gels and visualized by autoradiography. Lanes 1 and 2, undigested samples from complex C irradiated in the absence and presence of excess spermine, respectively. Lanes 3 to 6, samples hybridized (lane 3) with cDNAs 1962–1973 and 2159–2169, (lane 4) with cDNAs 2159–2169 and 2350–2360, (lane 5) with cDNAs 2350–2360 and 2568–2578, (lane 6) with cDNAs 2568–2578 and 2781–2791, and then digested with RNase H. Numbers indicated to the left of the gel correspond to the sizes (nt) of RNA markers. The region encompassing domain V of 23S rRNA, appears to be susceptible to ABA-spermine cross-linking.
The precise position of the cross-linking sites has been determined by primer extension analysis, making use of the fact that ABA-spermine attachment to a nucleoside of 23S rRNA acts as a barrier for reverse transcriptase (32). The site of cross-linking can, therefore, be localized exactly from the size of the reverse transcription product. The analysis is focused on the central loop of domain V of 23S rRNA, to which CAM binds. Representative autoradiograms are shown in Figure 6. The modified nucleosides by ABA-spermine are summarized in Figure 7. As shown, reverse transcriptase normally stops at a single nucleoside. Sometimes, primer extension produces ‘stuttering’ at a modified nucleoside, resulting in a strong 3′ band and a weaker 5′ band, as in the cases of A2600 and C2601 (Fig. 6B). However, for each of the multiple bands in Figure 6A, the band intensities are equal, suggesting that all the adjacent nucleosides are modified.
Figure 6.
Gel electrophoresis of products resulting from ABA-spermine photoincorporation into complex C, as monitored by primer extension analysis. The primers for reverse transcription were complementary to 23S rRNA positions (A) 2560–2576, and (B) 2697–2681. Positions at 10-nt intervals, read from sequencing products (lanes U, A, G and C), are shown in the left margin of each panel. Lane 1, untreated complex C; lane 2, complex C photolabeled with 100 µM ABA-spermine; lane 3, complex C photolabeled with 100 µM ABA-spermine in the presence of a 250-fold excess of spermine; lane 4, complex C modified by DMS; lane 5, complex C photolabeled with 100 µM ABA-spermine, and then modified by DMS. The stops of reverse transcriptase reaction are indicated in the right margin of each panel by arrows. SPM, spermine; DMS, dimethyl sulfate. Nucleosides in the central loop of domain V and in helices projecting from this loop (H89, H90 and H93) constitute preferred cross-linking sites for ABA-spermine. Upon ABA-spermine cross-linking, these regions undergo alterations in their reactivity towards DMS.
Figure 7.
Summary of ABA-spermine cross-linking sites in the central region of domain V of E.coli 23S rRNA. Cross-linking sites in a secondary structure model (cited at http://www.ma.icmb.utexas.edu) are marked with green arrows. Sites sensitive to divalent metal ion-catalyzed hydrolysis (38) are indicated by red arrows. Sites implicated in chloramphenicol binding (17–19) are shown by blue letters. ABA-spermine cross-linking sites and metal binding sites do not always coincide. Although the region encompassing nucleosides 2057–2062 constitutes a primary target for chloramphenicol binding, no ABA-spermine cross-linking is detected within this region.
Chemical probing with DMS indicates that spermine cross-linking alters the reactivity of certain nucleosides in the PTase loop towards DMS. Namely, complex C modified by ABA-spermine appears 12 positions in the PTase loop (A2453, C2475, A2476, A2497-C2499, C2501, A2518, A2564, C2573, C2575, C2610) of increased reactivity, not coinciding with ABA-spermine cross-linking sites. This finding suggests that, upon ABA-spermine cross-linking, the central loop of domain V undergoes alterations in its tertiary structure. Despite these alterations, the sedimentation profile of complex C remains unchanged, as detected by a previous study (31).
DISCUSSION
Most of the kinetic studies of CAM interaction with ribosomes, often contradictory, have been performed in the past under the prevalent notion that the complex of ribosome with the drug is formed via a simple equilibrium. Therefore, we were prompted toward a re-examination of the inhibition of peptide bond formation by CAM, by conducting our study under more physiological buffer conditions and by following a kinetic analysis allowing us to have a complete picture of the entire course of the reaction.
The results presented in this study demonstrate that CAM derives its potency through competition with the acceptor substrate. In fact, the new finding of our work is not the characterization of the partner with which CAM competes, but the observation that CAM behaves as a slow-binding inhibitor. The apparent association rate constant of CAM binding, (k6 + k7)/Ki, at 6 mM Mg2+ and 100 mM NH4+ equals 1.7 × 104 M–1 s–1, a value much lower than the upper limit of 106 M–1 s–1 set for the classification of a drug as a slow-binding inhibitor (34). This conclusion is also supported by the value of k7, which is less than that measured for the forward rate constant, k6. The observation that (kobs)o is enhanced by increasing concentrations of CAM (Fig. 1) precludes a binding mechanism of the type C + I ⇄ C*I. Corroborative evidence also comes from the plots shown in Figure 4; if a single-step mechanism could exist, the plots should be linear (34). Consequently, our results suggest that, upon CAM binding, complex C participates in conformational changes described by a two-step mechanism in which a fast association–dissociation equilibrium is followed by a slower rearrangement step which falls in the minute time range. A similar mechanism has been postulated by Langlois et al. (35). By studying the effect of CAM binding kinetics of a fluorescent derivative of erythromycin, F-Ery, with E.coli ribosomes, these authors suggested that CAM reacts rapidly with the 70S·F-Ery ribosomal complex to form an intermediate encounter complex, which is then isomerized to a final product, CAM·70S·F-Ery, by a slow, non-reversible and unimolecular process. However, they were not able to investigate this mechanism in more detail because the fluorescence change for this process was very small. Nevertheless, there is also evidence for noncompetitive (20) or mixed-noncompetitive mode of action (3,5). However, in these studies, the data have been obtained by pre-incubating ribosomes (the enzyme) with CAM and starting the reaction by the addition of puromycin (the substrate). Under these conditions, the slow-onset inhibition theory (34) predicts that the equation describing the initial first-order rate constant, (kobs)o, is
Double-reciprocal plots of this equation at different concentrations of inhibitor are intersected at a common point above the negative 1/[S] axis and, therefore, could be misinterpreted as indicating mixed-noncompetitive inhibition. This may explain the fact that for many years it could not be settled whether CAM was indeed a competitive inhibitor, as suggested by the structural similarity of CAM with the 3′-terminus of aminoacyl-tRNA.
The linearity of the slope replots shown in Figure 3 suggests that, under the experimental conditions employed, only one molecule of CAM is involved in the mechanism of inhibition; if more than one molecule participates, these plots should be parabolic. This observation is consistent with published crystallographic data (16). In fact, this is an unexpected finding, considering the size of CAM as well as the number of 23S rRNA nucleosides that have an impact on CAM binding. The situation becomes more complicated, if we take into account the fact that a peptidyl analogue of CAM, Gly-Phe-CAM, is characterized by non-linear slope replots (7). Since the stoichiometry of CAM binding was deduced by kinetic analysis, rather than from a real measurement of the amount of bound CAM, we cannot exclude the existence of additional CAM binding sites on ribosomal complex C. If they actually exist, they should be either mutually exclusive or not involved in the mechanism of inhibition. Also, we cannot exclude the possibility of additional sites of low affinity, operating at much higher concentrations of CAM.
According to Ki values, the potency of CAM at 6 mM Mg2+ is 2- to 4-fold lower than that previously found at 10 mM Mg2+ (3,5). Such a weakening of CAM potency upon decrease in optimal Mg2+ concentration, has also been observed in a mammalian protein-synthesizing system (21). The affinity of complex C for CAM is restored by addition of spermine or by pre-labeling of complex C with ABA-spermine (Table 1). The observed increase in CAM affinity corresponds to a free-energy difference (ΔG°) of about 3.5 kJ/mol. This implies that spermine exerts its beneficial action by lowering the entropic cost of CAM binding by pre-organizing its binding site. In contrast, the subsequent slow isomerization step does not depend on spermine, and seems to result from conformational changes induced by CAM. Positive or negative effects of polyamines on the interaction of other antibiotics with ribosomes have been previously pointed out (26,36). Spermine was chosen to participate in our experimental system, since it is the most effective among the naturally occurring polyamines, both in modulating the ribosomal functions and in decreasing the Mg2+ requirements for protein synthesis (37). Except from spermine, spermidine is also essential for optimal translational efficiency and accuracy (25). The concomitant presence of spermidine does not qualitatively influence the effect of spermine on the mechanism of CAM action (data not shown), and, since it enhances the complexity of cross-linking results, its use in the polyamine buffer was avoided.
The detailed knowledge of polyamine binding sites on ribosomes is a prerequisite for formulating a hypothesis explaining the effect of polyamines on the mechanism of CAM action. Progress in identifying binding sites for polyamines in 30S ribosomal subunits from E.coli has been made by affinity labeling techniques using photoreactive analogues of spermine (31,32). One of these analogues, ABA-spermine, was applied in the present study for mapping polyamine binding sites in the V-loop of 23S rRNA, to which CAM binds. Evidently, the central loop of domain V is a target of cross-linking by ABA-spermine (Fig. 7). It has been found that this region is also sensitive to divalent metal ion-catalyzed hydrolysis (38), a fact being in agreement with crystallographic data (16,23). Nevertheless, ABA-spermine cross-linking and metal binding sites do not always coincide, an observation supporting the notion that structural and functional changes of ribosomes induced by polyamines may be different from those induced by divalent metal ions. In the central loop of domain V, there are two regions of ABA-spermine cross-linking; a long stretch of strong cross-links around A2451 (nt 2449–2452) and a second weaker site at position U2506. From a structural and functional point of view, the importance of these labeled positions in CAM binding is beyond doubt. Various experimental approaches, including footprinting (17,18) and mutational analyses (19), hybridization with oligodeoxy-ribonucleotide probes (39), cross-linking techniques (8,10 and references therein) and X-ray crystallography studies (16,23), have demonstrated that both regions are located in the heart of the PTase center and are implicated in the formation of putative monovalent or Mg2+ ion binding sites involved in the stabilization of the catalytic center and the binding of CAM. For instance, the 3-OH group of CAM interacts with the 4-O group of U2506 through a Mg2+ ion that coordinates both groups (16). Nevertheless, at the resolution level of this study, the possibility of Mg2+ ion being either monovalent or a polyamine cannot be excluded. Additional ABA-spermine cross-linking sites, located in proximity to the above- mentioned positions, appear within helix H89, in the bulged loop of helix H90, and in helix H93. As far as helix H89 is concerned, it is known that hybridization of an oligodeoxy-ribonucleotide probe complementary to bases 2468–2482 is increased by 14% upon binding of CAM (39). According to the authors of this study, CAM binding induces conformational changes in the PTase center, probably implicated in the drug inhibitory effects. Crystallographic data have demonstrated that helix H89 runs in parallel to the acceptor arm of A-site bound tRNA, making a minor-groove interaction with its T-stem (40). Nucleosides in this region are also implicated in the fidelity of protein synthesis (19), a ribosomal function that is affected by both polyamines (25) and CAM (14). Another strong ABA-spermine cross-linking occurs in the bulged loop of helix H90, at A2572. This nucleoside exhibits a pH-dependent DMS reactivity (24) and contacts with the T-stem of A-site bound tRNA (40). Mutations at this position have strong effects on PTase activity (19). Next to this position, C2573 is very susceptible to metal ion-catalysed hydrolysis (38), and probably constitutes a general cation binding site. A cluster of cross-links localized at the base of helix H93, is in close proximity to position G2583 that is implicated in the sensitivity of ribosomes towards CAM and in the binding of aminoacyl-tRNA to the PTase center (41), and to position U2585 that is susceptible to Pb2+ cleavage (38). On the opposite strand of helix H93, primer extension produces ‘stuttering’ at nucleoside 2601 yielding a weaker 5′ band and a stronger 3′ band, a fact suggesting that cross-linking probably occurs at the latter position (Fig. 6B). Next to this site, there is a bulged nucleoside, A2602, that is positioned in the heart of PTase center and plays a critical role in aminoacyl-tRNA binding, RF1 binding, and in peptide release during termination (13). In addition, A2602 is one among several nucleosides in V-loop which is subjected to stereochemical changes upon CAM binding (42). In combination, these observations reveal the interdependence of CAM effects on protein biosynthesis and polyamine environment.
Although the X-ray structure of D.radiodurans 50S ribosomal subunits complexed with CAM has failed to detect any direct interaction of CAM with ribosomal proteins (16), reconstitution experiments and affinity-labeling studies (15) revealed that the drug also binds to proteins L16 and S3. It should be mentioned that both proteins are labeled by ABA-spermine (31), a fact further supporting the possibility of polyamine participation in CAM binding to ribosomes.
In conclusion, our results demonstrate that CAM behaves as a slow-binding inhibitor of peptide bond formation, blocking the accommodation of the 3′-terminus of aminoacyl-tRNA to the ribosomal catalytic cavity. Polyamines facilitate the binding of CAM to ribosome, probably by forming a connecting bridge between the drug and the ribosome. Alternatively, attachment of polyamines to ribosome may induce alterations in ribosomal conformation which favor CAM binding. Consistently, the central loop of domain V of 23S rRNA tends to adopt an apparent ‘open’ tertiary structure upon ABA-spermine photoincorporation, as indicated by DMS-protection experiments.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dennis Synetos for the critical review of the manuscript and Knud Nierhaus for providing E.coli CAN20–12E strain. This work was supported by a grant (99ED-605) from the General Secretariat of Research and Technology, Ministry of Development of Greece and the European Social Fund, and by a grant (E/108/DY2b/178) from the Ministry of Health.
APPENDIX
In Scheme 1, complex C is inactivated by conversion to C′ and by reacting with CAM. In deriving the corresponding equations, it is assumed that the equilibria C + S ⇄ CS and C + I ⇄ CI are established rapidly, while the unimolecular change of CI to C*I is established relatively slowly. Additionally, the value of k7 might be less than that of k6 for appreciable formation of C*I.
Under these assumptions, one can write
From the balance equation, [Co] = [C] + [CS] + [P] +[C*I], and from equations A1, A2, A3 and A4, it follows that
where
Equation A5 can be written also as
where:
By integration, equation A7 gives
where C is the integration constant.
As time, t, approaches zero, there is no C*I formation, but the equilibria C+S ⇄ CS and C+I ⇄ CI are in place. Thus, for t = 0
and
Substitution of equation A11 into equation A9 gives
From the relationship given in equation A8, it follows that
where:
and (kobs)s represents the kobs at the late phase of the puromycin reaction. Substitution of equations A10 and A13 into equation A12 gives
or
By integration, equation A15 gives
The curve corresponding to equation A16 has an asymptote given by equation A17
Consequently, the apparent equilibration rate constant, k′, for the attainment of equilibrium between complex C and CAM can be determined from the intersection point of the two linear parts of the corresponding progress curve. From the plot of k′ versus [CAM] at each concentration of puromycin, one can obtain by non-linear regression the rate constant values k6 and k7 (Microcal Origin, Version: 6.00, provided by Microcal Software, Inc.).
REFERENCES
- 1.Wareham D.W. and Wilson,P. (2002) Chloramphenicol in the 21st century. Hosp. Med., 63, 157–161. [DOI] [PubMed] [Google Scholar]
- 2.Pestka S. (1970) Formation of transfer ribonucleic acid-ribosome complexes. VII. Survey of the effect of antibiotics on N-acetylphenylalanyl puromycin formation: possible mechanism of chloramphenicol action. Arch. Biochem. Biophys., 136, 80–88. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Muñoz R. and Vazquez,D. (1973) Kinetic studies of peptide bond formation. Effect of chloramphenicol. Mol. Biol. Rep., 1, 75–79. [DOI] [PubMed] [Google Scholar]
- 4.Bhuta P., Chung,H.L., Hwang,J.-S. and Žemlička,J. (1980) Analogues of chloramphenicol: circular dichroism spectra, inhibition of ribosomal peptidyltransferase, and possible mechanism of action. J. Med. Chem., 23, 1299–1305. [DOI] [PubMed] [Google Scholar]
- 5.Drainas D., Kalpaxis,D.L. and Coutsogeorgopoulos,C. (1987) Inhibition of ribosomal peptidyltransferase by chloramphenicol: kinetic studies. Eur. J. Biochem., 164, 53–68. [DOI] [PubMed] [Google Scholar]
- 6.Kalpaxis D.L. and Coutsogeorgopoulos,C. (1989) Type of inhibition of peptide bond formation by chloramphenicol depends on the temperature and the concentration of ammonium ions. Mol. Pharmacol., 36, 615–619. [PubMed] [Google Scholar]
- 7.Michelinaki M., Mamos,P., Coutsogeorgopoulos,C. and Kalpaxis,D.L. (1997) Aminoacyl and peptidyl analogs of chloramphenicol as slow-binding inhibitors of ribosomal peptidyltransferase: a new approach for evaluating their potency. Mol. Pharmacol., 51, 139–146. [DOI] [PubMed] [Google Scholar]
- 8.Steiner G., Kuechler,E. and Barta,A. (1988) Photo-affinity labelling at the peptidyl transferase centre reveals two different positions for the A- and P-sites in domain V of 23S rRNA. EMBO J., 7, 3949–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rheinberger H.-J. and Nierhaus,K.H. (1990) Partial release of AcPhe-Phe-tRNA from ribosomes during poly(U)-dependent poly(Phe) synthesis and the effects of chloramphenicol. Eur. J. Biochem., 193, 643–650. [DOI] [PubMed] [Google Scholar]
- 10.Kirillov S.V., Porse,B.T. and Garrett,R.A. (1999) Peptidyl transferase antibiotics perturb the relative positioning of the 3′-terminal adenosine of P/P′-site-bound tRNA and 23S rRNA in the ribosome. RNA, 5, 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caskey C.T. and Beaudet,A.L. (1971) Antibiotic inhibitors of peptide termination. In Munoz,E., Garcias-Fernandez,F. and Vazquez,D. (eds), Molecular Mechanisms of Antibiotic Action on Protein Biosynthesis and Membranes. Elsevier, Amsterdam, pp. 326–336. [Google Scholar]
- 12.Lin A.H., Murray,R.W., Vidmar,T.J. and Marotti,K.R. (1997) The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob. Agents Chemother., 41, 2127–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polacek N., Gomez,M.J., Ito,K., Xiong,L., Nakamura,Y. and Mankin,A. (2003) The critical role of the universally conserved A2602 of 23S ribosomal RNA in the release of the nascent peptide during translation termination. Mol. Cell, 11, 103–112. [DOI] [PubMed] [Google Scholar]
- 14.Thompson J., O’Connor,M., Mills,J. and Dahlberg,A. (2002) The protein synthesis inhibitors, oxazolidones and chloramphenicol, cause extensive translational inaccuracy in vivo. J. Mol. Biol., 322, 273–279. [DOI] [PubMed] [Google Scholar]
- 15.Pongs O. (1979) Chloramphenicol. In Hahn,F.E. (ed.), Antibiotics. Springer-Verlag, New York, Vol. V, pp. 26–42. [Google Scholar]
- 16.Schlünzen F., Zarivach,R., Harms,J., Basham,A., Tocilj,A., Albrecht,R., Yonath,A. and Franceschi,F. (2001) Structural basis for the interaction of antibiotics with the peptidyl transferase center in eubacteria. Nature, 413, 814–821. [DOI] [PubMed] [Google Scholar]
- 17.Moazed D. and Noller,H.F. (1987) Chloramphenicol, erythromycin, carbomycin and vernamycin B overlapping sites in the peptidyl transferase region of 23S ribosomal RNA. Biochimie, 69, 879–884. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Fonseca C., Amils,R. and Garrett,R.A. (1995) Fine structure of the peptidyl transferase center on 23S-like rRNAs deduced from chemical probing of antibiotic-ribosome complexes. J. Mol. Biol., 247, 224–235. [DOI] [PubMed] [Google Scholar]
- 19.Triman K.L. (1999) Mutational analysis of 23S ribosomal RNA structure and function in Escherichia coli. Adv. Genet., 41, 157–195. [DOI] [PubMed] [Google Scholar]
- 20.Pestka S. (1974) The use of inhibitors in studies on protein synthesis. Methods Enzymol., 30, 261–282. [DOI] [PubMed] [Google Scholar]
- 21.Armentrout S.A. and Weisberger,A.S. (1967) Inhibition of directed protein synthesis by chloramphenicol: effect of magnesium concentration. Biochem. Biophys. Res. Commun., 26, 712–716. [DOI] [PubMed] [Google Scholar]
- 22.Vogel Z., Vogel,T., Zamir,A. and Elson,D. (1971) Correlation between the peptidyl transferase activity of the 50S ribosomal subunit and the ability of the subunit to interact with antibiotics. J. Mol. Biol., 60, 339–346. [DOI] [PubMed] [Google Scholar]
- 23.Nissen P., Hansen,J., Ban,N., Moore,P.B. and Steitz,T.A. (2000) The structural basis of ribosome activity in peptide bond synthesis. Science, 289, 920–930. [DOI] [PubMed] [Google Scholar]
- 24.Bayfield M.A., Dahlberg,A.E., Schulmeister,U., Dorner,S. and Barta,A. (2001) A conformational change in the ribosomal peptidyl transferase center upon active/inactive transition. Proc. Natl Acad. Sci. USA, 98, 10096–10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartetzko A. and Nierhaus,K.H. (1988) Mg2+/NH4+/polyamine system for polyuridine-dependent polyphenylalanine synthesis with near in vivo characteristics. Methods Enzymol., 164, 650–658. [DOI] [PubMed] [Google Scholar]
- 26.Teraoka H. and Tanaka,K. (1973) Effect of spermine on the binding of erythromycin to Escherichia coli ribosomes and the peptidyl-transfer reaction. Eur. J. Biochem., 33, 578–583. [DOI] [PubMed] [Google Scholar]
- 27.Liberman K.R. and Dahlberg,A.E. (1995) Ribosome-catalyzed peptide-bond formation. Prog. Nucleic Acids Res. Mol. Biol., 50, 1–23. [PubMed] [Google Scholar]
- 28.Clark C.T., Swank,R.A., Morgan,J.E., Basu,H. and Matthews,H.R. (1991) Two new photoaffinity polyamines appear to alter the helical twist of DNA in nucleosome core particles. Biochemistry, 30, 4009–4020. [DOI] [PubMed] [Google Scholar]
- 29.Kalpaxis D.L., Theocharis,D.A. and Coutsogeorgopoulos,C. (1986) Kinetic studies on ribosomal peptidyltransferase. The behaviour of the inhibitor blasticidin S. Eur. J. Biochem., 154, 267–271. [DOI] [PubMed] [Google Scholar]
- 30.Drainas D. and Kalpaxis,D.L. (1994) Bimodal action of spermine on ribosomal peptidyltransferase at low concentration of magnesium ions. Biochim. Biophys. Acta, 1208, 55–64. [DOI] [PubMed] [Google Scholar]
- 31.Amarantos I., Xaplanteri,M.A., Choli-Papadopoulou,T. and Kalpaxis,D.L. (2001) Effects of two photoreactive spermine analogues on peptide bond formation and their application for labeling proteins in Escherichia coli functional ribosomal complexes. Biochemistry, 40, 7641–7650. [DOI] [PubMed] [Google Scholar]
- 32.Amarantos I., Zarkadis,I.K. and Kalpaxis,D.L. (2002) The identification of spermine binding sites in 16S rRNA allows interpretation of the spermine effect on ribosomal 30S subunit functions. Nucleic Acids Res., 30, 2832–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalpaxis D.L. and Drainas,D. (1992) Effect of spermine on peptide-bond formation, catalyzed by ribosomal peptidyltransferase. Mol. Cell. Biochem., 115, 19–26. [DOI] [PubMed] [Google Scholar]
- 34.Morrison J.B. and Walsh,C.T. (1988) The behavior and significance of slow-binding enzyme inhibitors. Adv. Enzymol. Relat. Areas Mol. Biol., 61, 201–301. [DOI] [PubMed] [Google Scholar]
- 35.Langlois R., Cantor,C.R., Vince,R. and Pestka,S. (1977) Interaction between the erythromycin and chloramphenicol binding sites on the Escherichia coli ribosomes. Biochemistry, 16, 2349–2356. [DOI] [PubMed] [Google Scholar]
- 36.Diniello G.B., Algranati,I.D. and Goldemberg,S.H. (1998) Streptomycin bactericidal action is dependent on polyamine endogenous levels in Escherichia coli. Cell. Mol. Biol., 44, 521–526. [PubMed] [Google Scholar]
- 37.Tabor C.W. and Tabor,H. (1976) 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu. Rev. Biochem., 45, 285–306. [DOI] [PubMed] [Google Scholar]
- 38.Polacek N. and Barta,A. (1998) Metal ion probing of tRNAs: evidence for evolutionarily conserved divalent cation binding pockets. RNA, 4, 1282–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marconi R.T., Lodmell,J.S. and Hill,W.E. (1990) Identification of a rRNA/chloramphenicol interaction site within the peptidyltransferase center of the 50S subunit of the Escherichia coli ribosome. J. Biol. Chem., 265, 7894–7899. [PubMed] [Google Scholar]
- 40.Yusupov M.M., Yusupova,G.Z., Baucom,A., Lieberman,K., Earnest,T.N., Cate,J.H.D. and Noller,H.F. (2001) Crystal structure of the ribosome at 5.5 Å resolution. Science, 292, 883–896. [DOI] [PubMed] [Google Scholar]
- 41.Saarma U. and Remme,J. (1992) Novel mutants of 23S rRNA: characterization of functional properties. Nucleic Acids Res., 20, 3147–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porse B.T., Kirillov,S.V., Awayez,M.J., Ottenheijm,H.C.J. and Garrett,R.A. (1999) Direct crosslinking of the antitumor antibiotic sparsomycin, and its derivatives, to A2602 in the peptidyltransferase center of 23S-like rRNA within ribosome-tRNA complexes. Proc. Natl Acad. Sci. USA, 96, 9003–9008. [DOI] [PMC free article] [PubMed] [Google Scholar]