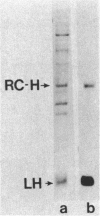
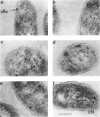
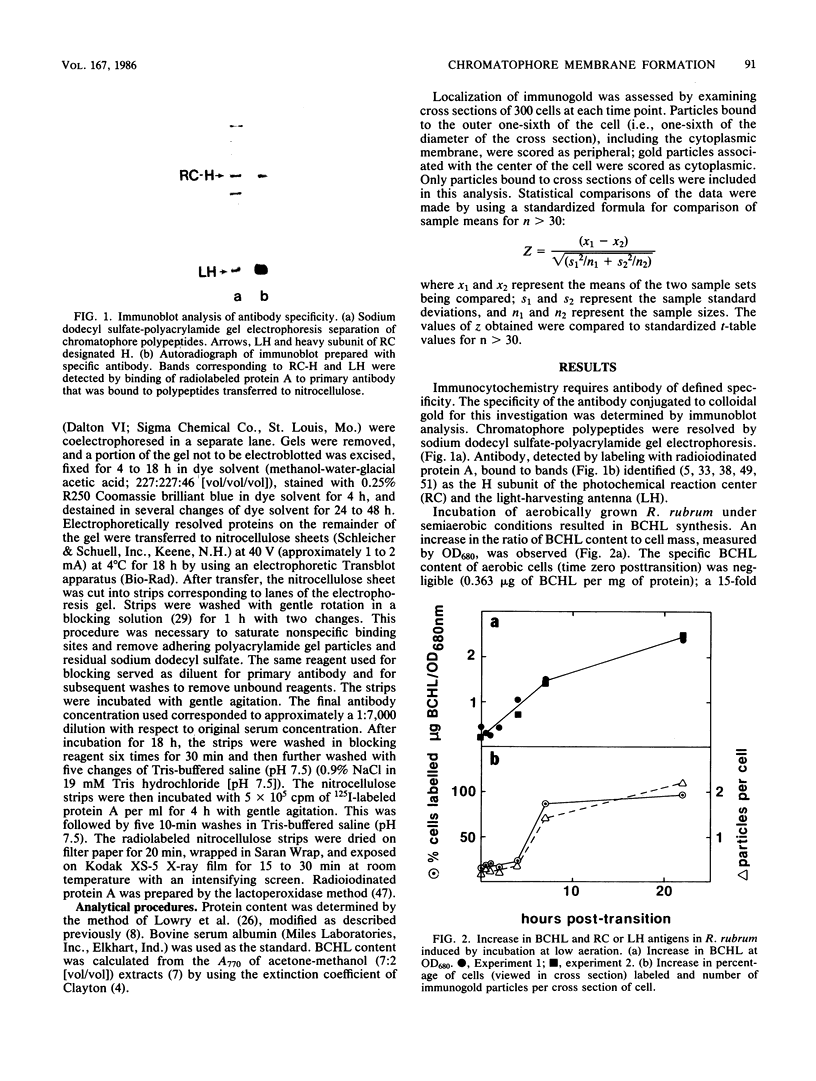
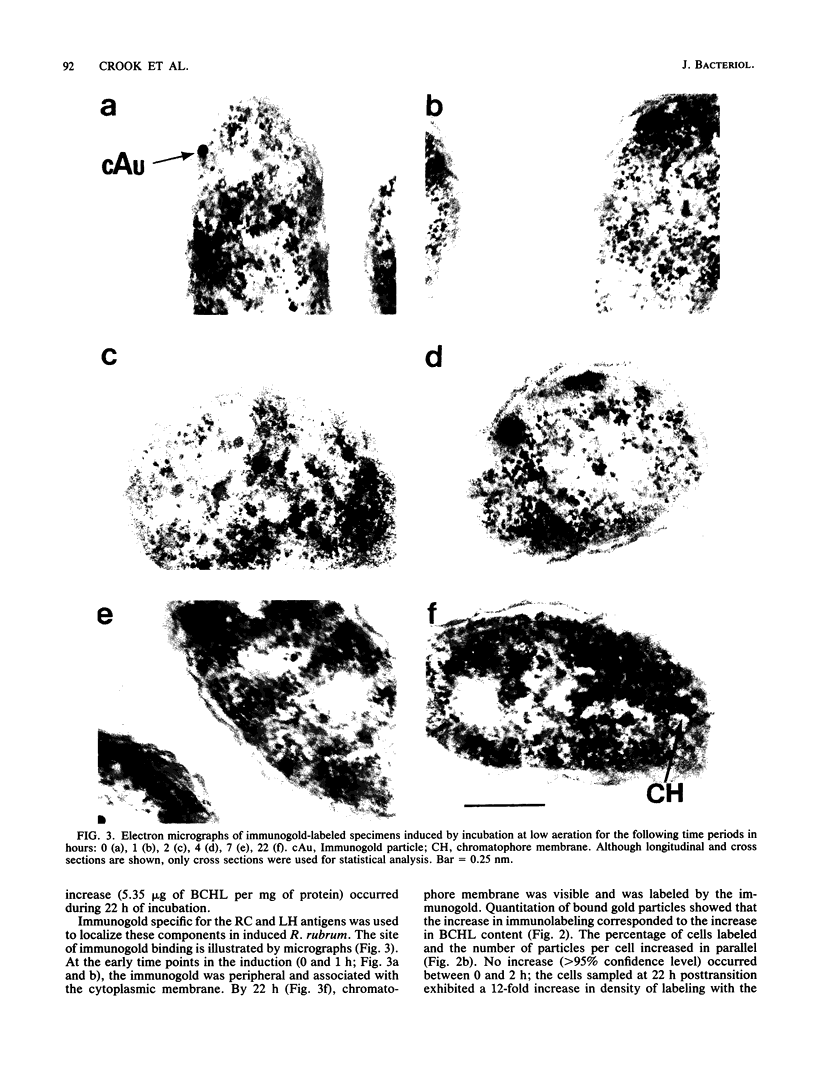
Abstract
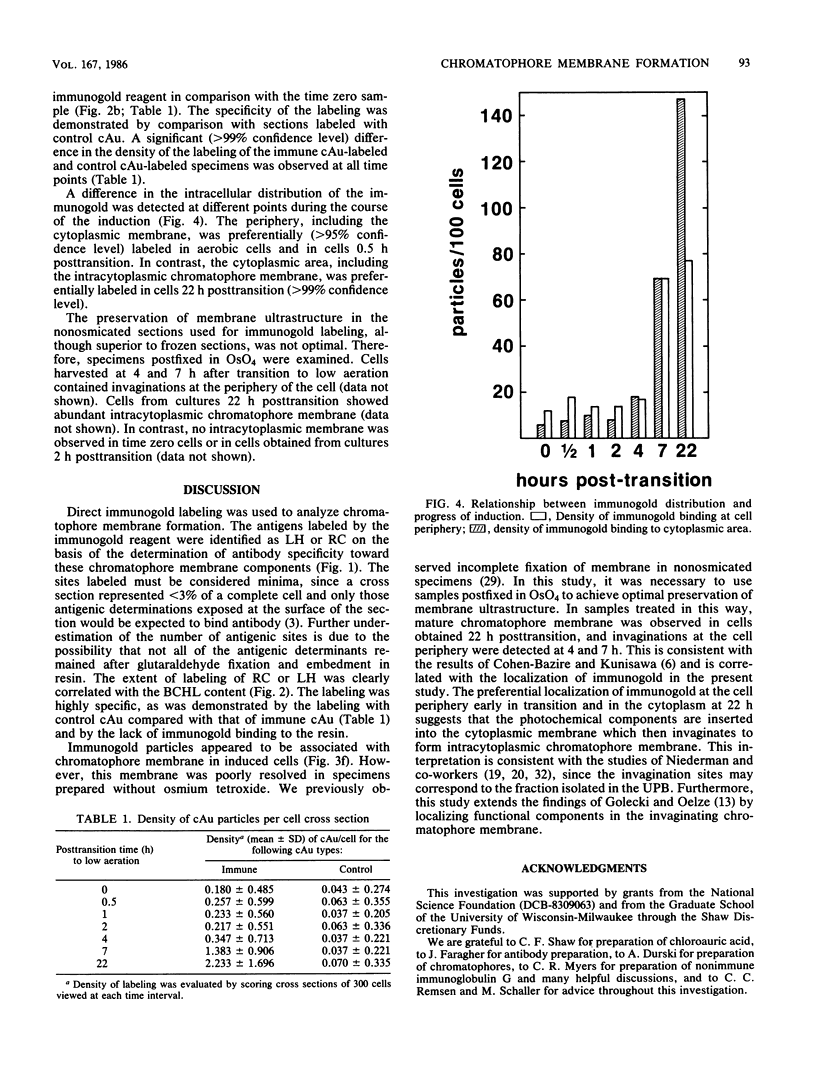
An immunocytochemical ultrastructural study of Rhodospirillum rubrum cultured under semiaerobic conditions was conducted to correlate the localization of functional components with membrane formation. R. rubrum is a facultatively phototrophic organism. Under reduced oxygen, this bacterium forms an intracytoplasmic chromatophore membrane that is the site of the photosynthetic apparatus. Immunogold techniques were used to localize intracellular protein antigens associated with the photosynthetic apparatus. Antibody, demonstrated by immunoblotting to be specific for the reaction center and light-harvesting photochemical components, was conjugated to colloidal gold particles and used for direct immunolabeling of fixed, sectioned specimens. Membrane invaginations appeared by 4 h after transition to induction conditions, and mature chromatophore membrane was abundant by 22 h. The occurrence of chromatophore membrane was correlated with bacteriochlorophyll a content and the density of the immunolabel. In uninduced (aerobic) cells and those obtained from cultures 0.5 h posttransition, the immunogold preferentially labeled the peripheral area of the cell. In contrast, in cells obtained after 22 h of induction, the central region of the cell was preferentially immunolabeled. These findings provided immunocytochemical evidence supporting the hypothesis that the chromatophore membrane is formed by invagination of the cytoplasmic membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E. A., Setter E., Lamed R. Organization and distribution of the cellulosome in Clostridium thermocellum. J Bacteriol. 1985 Aug;163(2):552–559. doi: 10.1128/jb.163.2.552-559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Carlemalm E., Kellenberger E. Capsule of Escherichia coli K29: ultrastructural preservation and immunoelectron microscopy. J Bacteriol. 1985 Jun;162(3):985–991. doi: 10.1128/jb.162.3.985-991.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., KUNISAWA R. The fine structure of Rhodospirillum rubrum. J Cell Biol. 1963 Feb;16:401–419. doi: 10.1083/jcb.16.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Collins M. L., Mallon D. E., Niederman R. A. Assessment of Rhodopseudomonas sphaeroides chromatophore membrane asymmetry through bilateral antiserum adsorption studies. J Bacteriol. 1980 Jul;143(1):221–230. doi: 10.1128/jb.143.1.221-230.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. L., Salton M. R. Solubility characteristics of Micrococcus lysodeikticus membrane components in detergents and chaotropic salts analyzed by immunoelectrophoresis. Biochim Biophys Acta. 1979 May 3;553(1):40–53. doi: 10.1016/0005-2736(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Elferink M. G., Hellingwerf K. J., Michels P. A., Seÿen H. G., Konings W. N. Immunochemical analysis of membrane vesicles and chromatophoresis of Rhodopseudomonas sphaeroides by crossed immunoelectrophoresis. FEBS Lett. 1979 Nov 15;107(2):300–307. doi: 10.1016/0014-5793(79)80395-6. [DOI] [PubMed] [Google Scholar]
- Goodman S. L., Hodges G. M., Trejdosiewicz L. K., Livingston D. C. Colloidal gold probes--a further evaluation. Scan Electron Microsc. 1979;(3):619–628. [PubMed] [Google Scholar]
- Guan T., Ghosh A., Ghosh B. K. Immunoelectron microscopic double labeling of alkaline phosphatase and penicillinase with colloidal gold in frozen thin sections of Bacillus licheniformis 749/C. J Bacteriol. 1985 Oct;164(1):107–113. doi: 10.1128/jb.164.1.107-113.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Rosset J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem. 1977 Apr;25(4):295–305. doi: 10.1177/25.4.323352. [DOI] [PubMed] [Google Scholar]
- Inamine G. S., Niederman R. A. Development and growth of photosynthetic membranes of Rhodospirillum rubrum. J Bacteriol. 1982 Jun;150(3):1145–1153. doi: 10.1128/jb.150.3.1145-1153.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine G. S., Van Houten J., Niederman R. A. Intracellular localization of photosynthetic membrane growth initiation sites in Rhodopseudomonas sphaeroides. J Bacteriol. 1984 May;158(2):425–429. doi: 10.1128/jb.158.2.425-429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Edwards H. H., Davis D., Rock C. O. Localization of acyl carrier protein in Escherichia coli. J Bacteriol. 1985 Apr;162(1):5–8. doi: 10.1128/jb.162.1.5-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka S., Yasuda Y., Tochikubo K. Ultrastructural localization of dipicolinic acid in dormant spores of Bacillus subtilis by immunoelectron microscopy with colloidal gold particles. J Bacteriol. 1985 Jun;162(3):1250–1254. doi: 10.1128/jb.162.3.1250-1254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lommen M. A., Takemoto J. Comparison, by freeze-fracture electron microscopy, of chromatophores, spheroplast-derived membrane vesicles, and whole cells of Rhodopseudomonas sphaeroides. J Bacteriol. 1978 Nov;136(2):730–741. doi: 10.1128/jb.136.2.730-741.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. R., Collins M. L. Identification of two distinct lactate dehydrogenases in Rhodospirillum rubrum. J Bacteriol. 1983 Mar;153(3):1562–1566. doi: 10.1128/jb.153.3.1562-1566.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C. R., Bergtrom G., Crook S. M., Boyer D. R., Collins M. L. Post-embedding direct immunogold detection of a protein antigen in insect tissue. J Histochem Cytochem. 1986 Feb;34(2):221–226. doi: 10.1177/34.2.3511140. [DOI] [PubMed] [Google Scholar]
- Myers C. R., Collins M. L. Cell-cycle-specific oscillation in the composition of chromatophore membrane in Rhodospirillum rubrum. J Bacteriol. 1986 Jun;166(3):818–823. doi: 10.1128/jb.166.3.818-823.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederman R. A., Mallon D. E., Langan J. J. Membranes of Rhodopseudomonas sphaeroides. IV. Assembly of chromatophores in low-aeration cell suspensions. Biochim Biophys Acta. 1976 Aug 13;440(2):429–447. doi: 10.1016/0005-2728(76)90076-1. [DOI] [PubMed] [Google Scholar]
- Niederman R. A., Mallon D. E., Parks L. C. Membranes of Rhodopseudomonas sphaeroides. VI. Isolation of a fraction enriched in newly synthesized bacteriochlorophyll alpha-protein complexes. Biochim Biophys Acta. 1979 Aug 7;555(2):210–220. doi: 10.1016/0005-2736(79)90161-5. [DOI] [PubMed] [Google Scholar]
- Noël H., Van der Rest M., Gingras G. Isolation and partial characterization of P870 reaction center complex from wild type Rhodospirillum rubrum. Biochim Biophys Acta. 1972 Aug 17;275(2):219–230. doi: 10.1016/0005-2728(72)90043-6. [DOI] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Oelze J. Proteins exposed at the surface of chromatophores of Rhodospirillum rubrum: the orientation of isolated chromatophores. Biochim Biophys Acta. 1978 Jun 2;509(3):450–461. doi: 10.1016/0005-2736(78)90239-0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo G. M., Van Etten J. L. Synthesis and localization of a development-specific protein in sclerotia of Sclerotinia sclerotiorum. J Bacteriol. 1985 Aug;163(2):696–703. doi: 10.1128/jb.163.2.696-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes P., Mitchell P., Moyle J. The polarity of proton translocation in some photosynthetic microorganisms. Eur J Biochem. 1969 Apr;8(3):450–454. doi: 10.1111/j.1432-1033.1969.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Sherwood J. E., Vasse J. M., Dazzo F. B., Truchet G. L. Development and trifoliin A-binding ability of the capsule of Rhizobium trifolii. J Bacteriol. 1984 Jul;159(1):145–152. doi: 10.1128/jb.159.1.145-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M. L., Bowers B., Cabib E. Formation of septum-like structures at locations remote from the budding sites in cytokinesis-defective mutants of Saccharomyces cerevisiae. J Bacteriol. 1985 May;162(2):763–767. doi: 10.1128/jb.162.2.763-767.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swafford J. R., Malloy P. J., Reeves H. C. Immunochemical localization of NADP-specific isocitrate dehydrogenase in Escherichia coli. Science. 1983 Jul 15;221(4607):295–296. doi: 10.1126/science.6344223. [DOI] [PubMed] [Google Scholar]
- Swafford J. R., Reeves H. C., Brandsch R. Localization of the enantiozymes of 6-hydroxy-nicotine oxidase in Arthrobacter oxidans by electron immunochemistry. J Bacteriol. 1985 Aug;163(2):792–795. doi: 10.1128/jb.163.2.792-795.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Tinglu G., Ghosh A., Ghosh B. K. Subcellular localization of alkaline phosphatase in Bacillus licheniformis 749/C by immunoelectron microscopy with colloidal gold. J Bacteriol. 1984 Aug;159(2):668–677. doi: 10.1128/jb.159.2.668-677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C., Noël H., Poirier L., Cloutier Y., Gingras G. Photoreaction center of photosynthetic bacteria. 1. Further chemical characterization of the photoreaction center from Rhodospirillum rubrum. Biochemistry. 1979 Oct 2;18(20):4301–4308. doi: 10.1021/bi00587a007. [DOI] [PubMed] [Google Scholar]
- Vos-Scheperkeuter G. H., Pas E., Brakenhoff G. J., Nanninga N., Witholt B. Topography of the insertion of LamB protein into the outer membrane of Escherichia coli wild-type and lac-lamB cells. J Bacteriol. 1984 Aug;159(2):440–447. doi: 10.1128/jb.159.2.440-447.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürrer H., Snozzi M., Hanselmann K., Bachofen R. Localisation of the subunits of the photosynthetic reaction centers in the chromatophore membrane of Rhodospirillum rubrum. Biochim Biophys Acta. 1977 May 11;460(2):273–279. doi: 10.1016/0005-2728(77)90213-4. [DOI] [PubMed] [Google Scholar]