Abstract
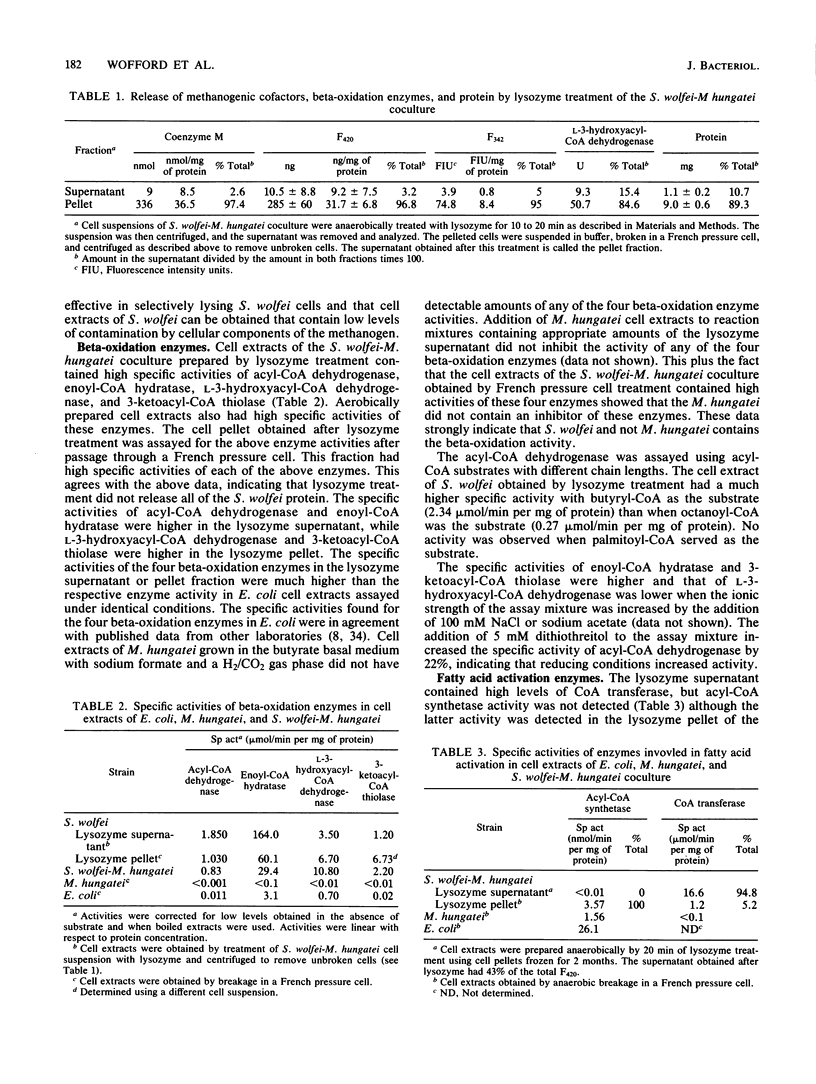
Syntrophomonas wolfei is an anaerobic fatty acid degrader that can only be grown in coculture with H2-using bacteria such as Methanospirillum hungatei. Cells of S. wolfei were selectively lysed by lysozyme treatment, and unlysed cells of M. hungatei were removed by centrifugation. The cell extract of S. wolfei obtained with this method had low levels of contamination by methanogenic cofactors. However, lysozyme treatment was not efficient in releasing S. wolfei protein; only about 15% of the L-3-hydroxyacyl-coenzyme A (CoA) dehydrogenase activity was found in the lysozyme supernatant. Cell extracts of S. wolfei obtained with this method had high specific activities of acyl-CoA dehydrogenase, enoyl-CoA hydratase, L-3-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-CoA thiolase. These activities were not detected in cell extracts of M. hungatei grown alone, confirming that these activities were present in S. wolfei. The acyl-CoA dehydrogenase activity was high when a C4 but not a C8 or C16 acyl-CoA derivative served as the substrate. S. Wolfei cell extracts had high CoA transferase specific activities and no detectable acyl-CoA synthetase activity, indicating that fatty acid activation occurred by transfer of CoA from acetyl-CoA. Phosphotransacetylase and acetate kinase activities were detected in cell extracts of S. wolfei, indicating that S. wolfei is able to perform substrate-level phosphorylation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGMEYER H. U., HOLZ G., KLOTZSCH H., LANG G. PHOSPHOTRANSACETYLASE AUS CLOSTRIDIUM KLUYVERI. ZUECHTUNG DES BACTERIUMS, ISOLIERUNG, KRISTALLISATION UND EIGENSCHAFTEN DES ENZYMS. Biochem Z. 1963;338:114–121. [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock J. F., Schulz H. Fatty acid oxidation complex from Escherichia coli. Methods Enzymol. 1981;71(Pt 100):403–411. doi: 10.1016/0076-6879(81)71051-6. [DOI] [PubMed] [Google Scholar]
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw R. A., Noyes B. E. L-3-hydroxyacyl coenzyme A dehydrogenase from pig heart muscle. EC 1.1.1.35 L-3-hydroxyacyl-CoA: NAD oxidoreductase. Methods Enzymol. 1975;35:122–128. doi: 10.1016/0076-6879(75)35147-1. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., McBride B. C., Wolfe R. S. Hydrogen-oxidizing methane bacteria. I. Cultivation and methanogenesis. J Bacteriol. 1968 Mar;95(3):1118–1123. doi: 10.1128/jb.95.3.1118-1123.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman P., Toms-Wood A., Wolfe R. S. Isolation and properties of a fluorescent compound, factor 420 , from Methanobacterium strain M.o.H. J Bacteriol. 1972 Oct;112(1):527–531. doi: 10.1128/jb.112.1.527-531.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirich L. D., Vogels G. D., Wolfe R. S. Proposed structure for coenzyme F420 from Methanobacterium. Biochemistry. 1978 Oct 31;17(22):4583–4593. doi: 10.1021/bi00615a002. [DOI] [PubMed] [Google Scholar]
- Fong J. C., Schulz H. Short-chain and long-chain enoyl-CoA hydratases from pig heart muscle. Methods Enzymol. 1981;71(Pt 100):390–398. doi: 10.1016/0076-6879(81)71049-8. [DOI] [PubMed] [Google Scholar]
- Genthner B. R., Davis C. L., Bryant M. P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981 Jul;42(1):12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmanis M. G., Gatenbeck S. Intermediary Metabolism in Clostridium acetobutylicum: Levels of Enzymes Involved in the Formation of Acetate and Butyrate. Appl Environ Microbiol. 1984 Jun;47(6):1277–1283. doi: 10.1128/aem.47.6.1277-1283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J. M., Smith P. H. Isolation of a Butyrate-Utilizing Bacterium in Coculture with Methanobacterium thermoautotrophicum from a Thermophilic Digester. Appl Environ Microbiol. 1985 Jun;49(6):1461–1466. doi: 10.1128/aem.49.6.1461-1466.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hii V., Courtright J. B. Induction of acyl coenzyme A synthetase and hydroxyacyl coenzyme A dehydrogenase during fatty acid degradation in Neurospora crassa. J Bacteriol. 1982 May;150(2):981–983. doi: 10.1128/jb.150.2.981-983.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic synthesis of the coenzyme A derivatives of long chain fatty acids. J Biol Chem. 1953 Sep;204(1):329–343. [PubMed] [Google Scholar]
- Kaspar H. F., Wuhrmann K. Kinetic parameters and relative turnovers of some important catabolic reactions in digesting sludge. Appl Environ Microbiol. 1978 Jul;36(1):1–7. doi: 10.1128/aem.36.1.1-7.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie R. I., Bryant M. P. Metabolic Activity of Fatty Acid-Oxidizing Bacteria and the Contribution of Acetate, Propionate, Butyrate, and CO(2) to Methanogenesis in Cattle Waste at 40 and 60 degrees C. Appl Environ Microbiol. 1981 Jun;41(6):1363–1373. doi: 10.1128/aem.41.6.1363-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P., Hespell R. B., Costerton J. W. Syntrophomonas wolfei gen. nov. sp. nov., an Anaerobic, Syntrophic, Fatty Acid-Oxidizing Bacterium. Appl Environ Microbiol. 1981 Apr;41(4):1029–1039. doi: 10.1128/aem.41.4.1029-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton B. 3-Ketoacyl-CoA thiolases of mammalian tissues. Methods Enzymol. 1975;35:128–136. doi: 10.1016/0076-6879(75)35148-3. [DOI] [PubMed] [Google Scholar]
- Moskowitz G. J., Merrick J. M. Metabolism of poly-beta-hydroxybutyrate. II. Enzymatic synthesis of D-(-)-beta hydroxybutyryl coenzyme A by an enoyl hydrase from Rhodospirillum rubrum. Biochemistry. 1969 Jul;8(7):2748–2755. doi: 10.1021/bi00835a009. [DOI] [PubMed] [Google Scholar]
- O'Brien W. J., Frerman F. E. Evidence for a complex of three beta-oxidation enzymes in Escherichia coli: induction and localization. J Bacteriol. 1977 Nov;132(2):532–540. doi: 10.1128/jb.132.2.532-540.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlies G., Fuchs G., Thauer R. K. Acetate thiokinase and the assimilation of acetate in methanobacterium thermoautotrophicum. Arch Microbiol. 1980 Dec;128(2):248–252. doi: 10.1007/BF00406167. [DOI] [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- Robinson J. A., Tiedje J. M. Nonlinear estimation of Monod growth kinetic parameters from a single substrate depletion curve. Appl Environ Microbiol. 1983 May;45(5):1453–1458. doi: 10.1128/aem.45.5.1453-1458.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe C. Acyl-CoA dehydrogenase from pig kidney. Methods Enzymol. 1981;71(Pt 100):366–374. doi: 10.1016/0076-6879(81)71046-2. [DOI] [PubMed] [Google Scholar]
- Weeks G., Shapiro M., Burns R. O., Wakil S. J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J Bacteriol. 1969 Feb;97(2):827–836. doi: 10.1128/jb.97.2.827-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin M. J. Lysis of Vibrio succinogenes by ethylenediamine-tetraacetic acid or lysozyme. J Bacteriol. 1966 May;91(5):1781–1786. doi: 10.1128/jb.91.5.1781-1786.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


