Abstract
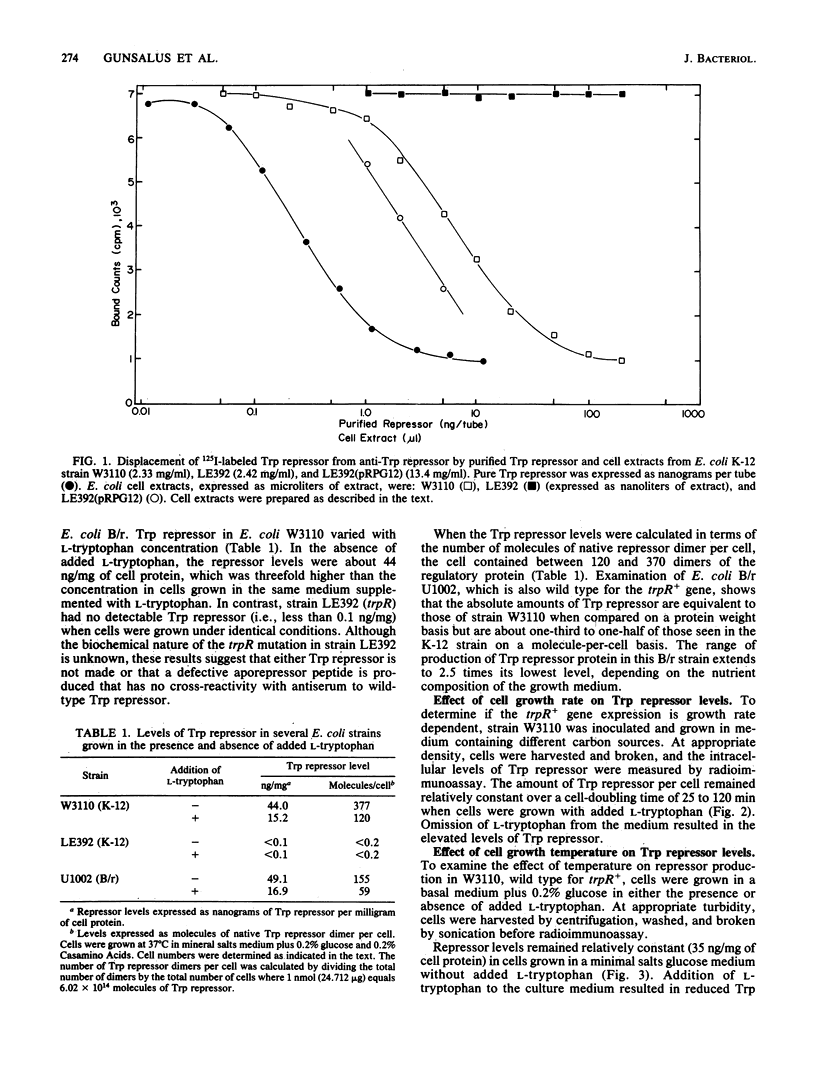
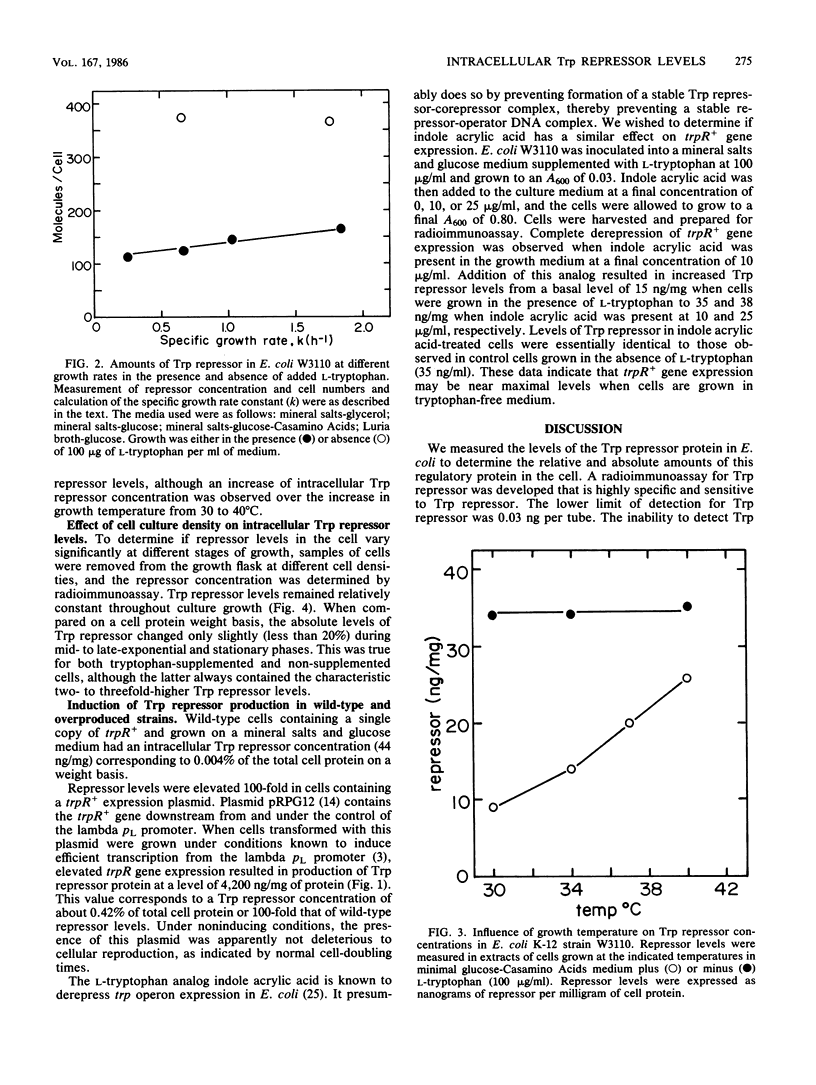
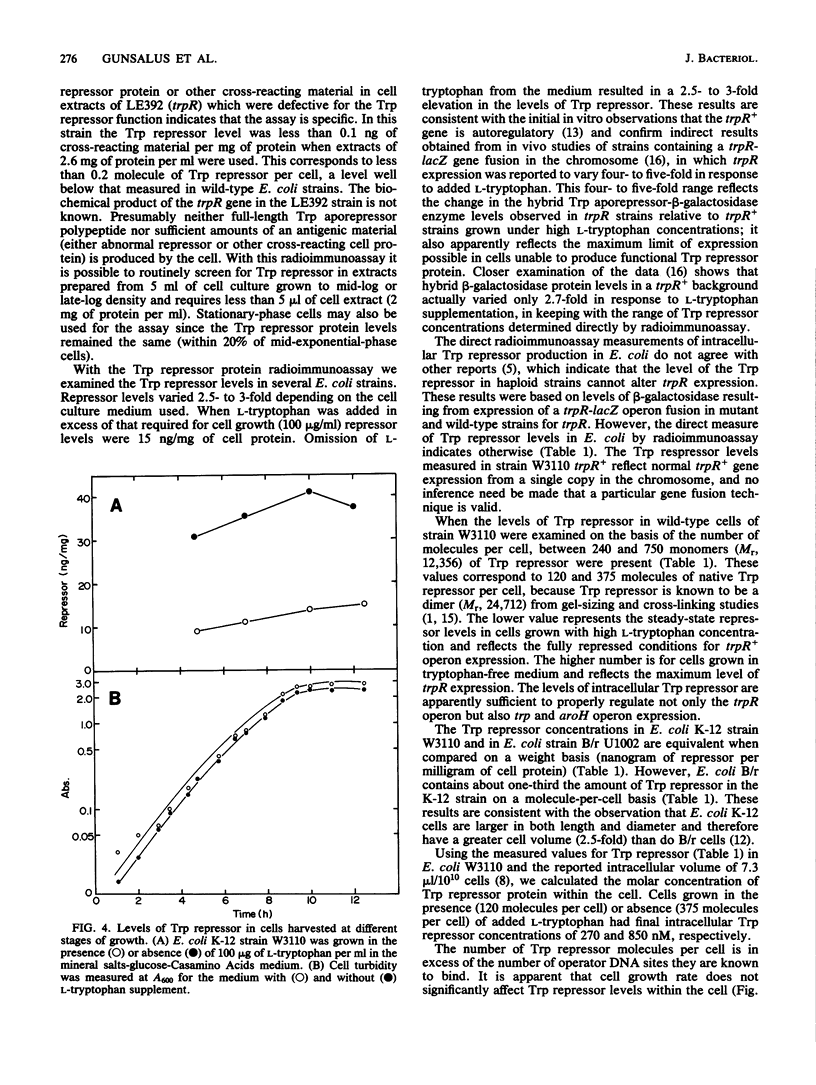
A radioimmunoassay for the Trp repressor protein of Escherichia coli was developed with antisera raised against purified Trp repressor protein. This assay was used to directly measure the intracellular Trp repressor content in several E. coli K-12 and B/r strains. Repressor levels varied from 2.5- to 3-fold in response to L-tryptophan concentration in the growth medium (15 to 44 ng of repressor per mg of protein). Neither cell growth rate nor culture age had a significant effect on repressor concentrations within the cell. Addition of L-tryptophan to the growth medium resulted in lowered intracellular levels of Trp repressor. The absolute amounts of native Trp repressor molecules per cell varied between 120 and 375 dimers in the presence and absence of L-tryptophan in the culture medium, respectively. Assuming an intracellular volume of 7.3 microliters/10(10) E. coli cells, the Trp repressor concentration varied from 270 to 850 nM in response to extracellular tryptophan levels. These findings represent the first direct measurements of Trp repressor levels in E. coli and confirm the autoregulatory nature of the trpR gene.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidson D. N., Bruce C., Gunsalus R. P. Interaction of the Escherichia coli trp aporepressor with its ligand, L-tryptophan. J Biol Chem. 1986 Jan 5;261(1):238–243. [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard H. U., Remaut E., Hershfield M. V., Das H. K., Helinski D. R., Yanofsky C., Franklin N. Construction of plasmid cloning vehicles that promote gene expression from the bacteriophage lambda pL promoter. Gene. 1979 Jan;5(1):59–76. doi: 10.1016/0378-1119(79)90092-1. [DOI] [PubMed] [Google Scholar]
- Bogosian G., Bertrand K., Somerville R. Trp repressor protein controls its own structural gene. J Mol Biol. 1981 Jul 15;149(4):821–825. doi: 10.1016/0022-2836(81)90361-2. [DOI] [PubMed] [Google Scholar]
- Bogosian G., Somerville R. L. Analysis in vivo of factors affecting the control of transcription initiation at promoters containing target sites for trp repressor. Mol Gen Genet. 1984;193(1):110–118. doi: 10.1007/BF00327423. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cover W. H., Martinez R. J., Rittenberg S. C. Permeability of the boundary layers of Bdellovibrio bacteriovorus 109J and its bdelloplasts to small hydrophilic molecules. J Bacteriol. 1984 Feb;157(2):385–390. doi: 10.1128/jb.157.2.385-390.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- GROSS J., ENGLESBERG E. Determination of the order of mutational sites governing L-arabinose utilization in Escherichia coli B/r bv transduction with phage Plbt. Virology. 1959 Nov;9:314–331. doi: 10.1016/0042-6822(59)90125-4. [DOI] [PubMed] [Google Scholar]
- Grossman N., Ron E. Z., Woldringh C. L. Changes in cell dimensions during amino acid starvation of Escherichia coli. J Bacteriol. 1982 Oct;152(1):35–41. doi: 10.1128/jb.152.1.35-41.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Yanofsky C. Nucleotide sequence and expression of Escherichia coli trpR, the structural gene for the trp aporepressor. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7117–7121. doi: 10.1073/pnas.77.12.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Zurawski G., Yanofsky C. Structural and functional analysis of cloned deoxyribonucleic acid containing the trpR-thr region of the Escherichia coli chromosome. J Bacteriol. 1979 Oct;140(1):106–113. doi: 10.1128/jb.140.1.106-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R. L., Yanofsky C. Trp aporepressor production is controlled by autogenous regulation and inefficient translation. Proc Natl Acad Sci U S A. 1982 May;79(10):3120–3124. doi: 10.1073/pnas.79.10.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Amber mutants of the trpR regulatory gene. J Mol Biol. 1969 Aug 28;44(1):185–193. doi: 10.1016/0022-2836(69)90413-6. [DOI] [PubMed] [Google Scholar]
- Pittard J., Camakaris J., Wallace B. J. Inhibition of 3-deoxy-d-arabinoheptulosonic acid-7-phosphate synthetase (trp) in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1242–1247. doi: 10.1128/jb.97.3.1242-1247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder W., Somerville R. L. Cloning the trpR gene. Mol Gen Genet. 1979 Nov;176(3):361–368. doi: 10.1007/BF00333098. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Yanofsky C. Interaction of the operator of the tryptophan operon with repressor. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3134–3138. doi: 10.1073/pnas.71.8.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yanofsky C. Tryptophan biosynthesis in Escherichia coli. Genetic determination of the proteins involved. JAMA. 1971 Nov 15;218(7):1026–1035. [PubMed] [Google Scholar]
- Zubay G., Morse D. E., Schrenk W. J., Miller J. H. Detection and isolation of the repressor protein for the tryptophan operon of Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1100–1103. doi: 10.1073/pnas.69.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Gunsalus R. P., Brown K. D., Yanofsky C. Structure and regulation of aroH, the structural gene for the tryptophan-repressible 3-deoxy-D-arabino-heptulosonic acid-7-phosphate synthetase of Escherichia coli. J Mol Biol. 1981 Jan 5;145(1):47–73. doi: 10.1016/0022-2836(81)90334-x. [DOI] [PubMed] [Google Scholar]


