Abstract
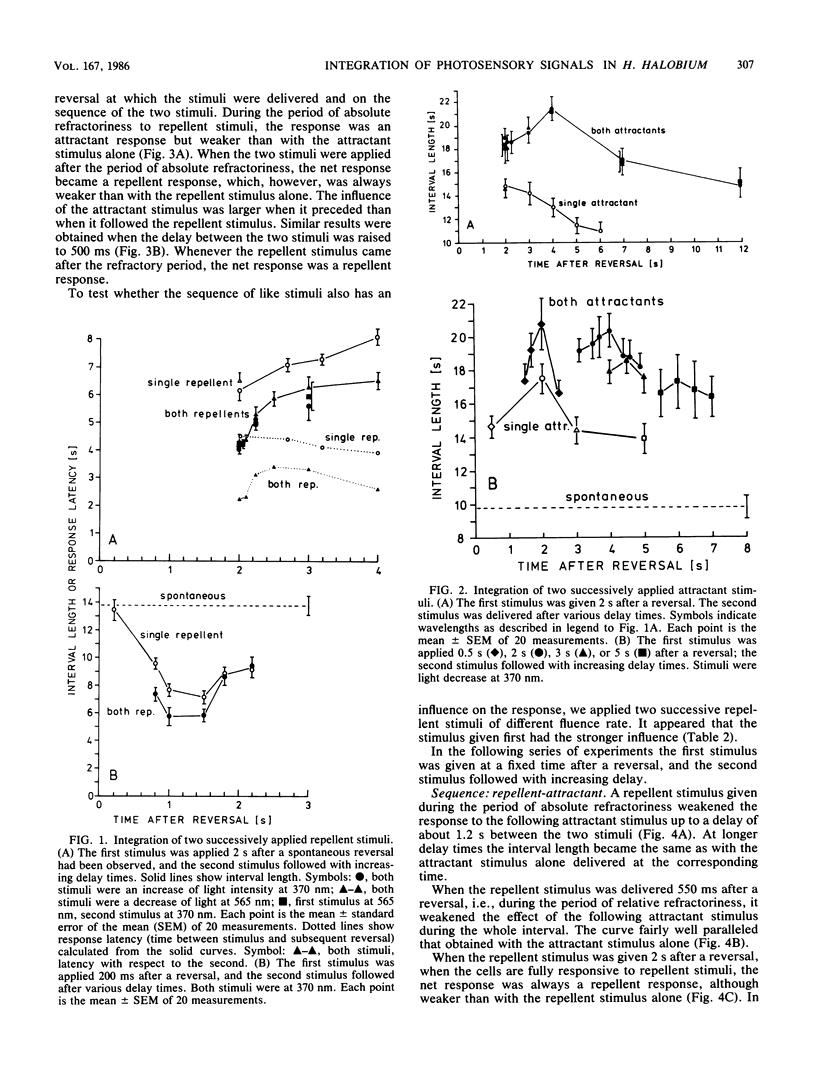
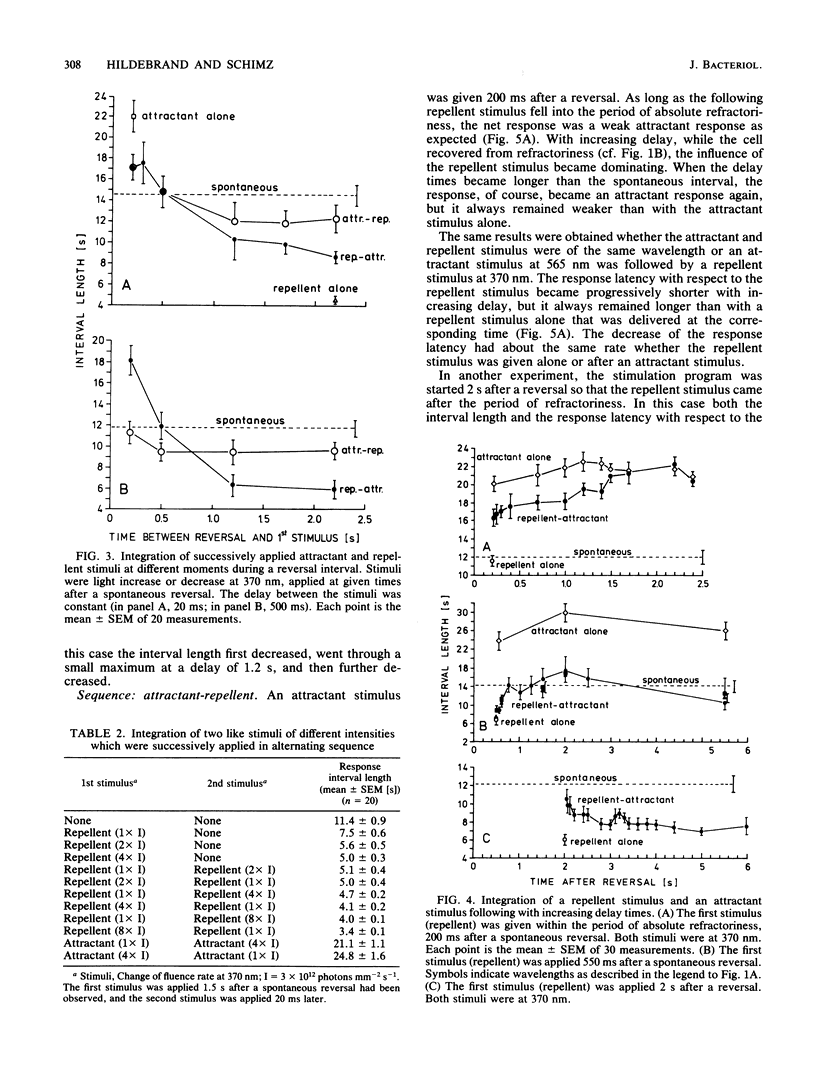
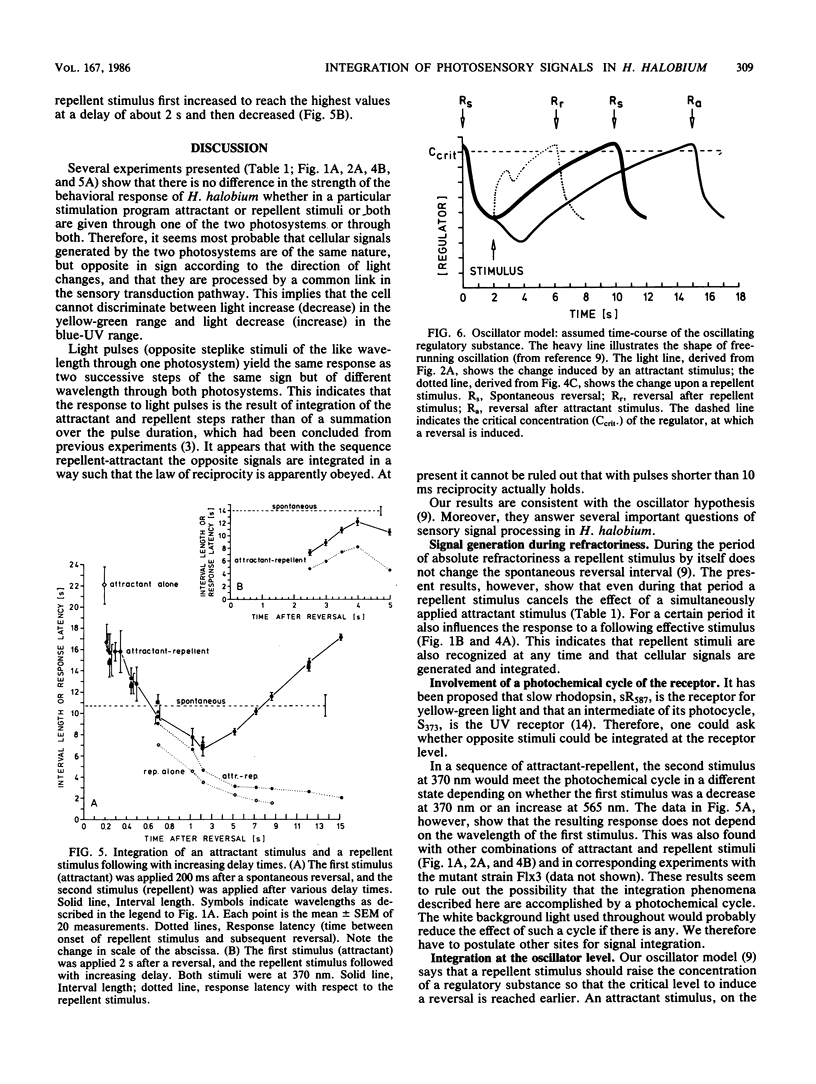
Stimulation of Halobacterium halobium through its sensory photosystems, PS 370 and PS 565, leads either to a prolonged or to a shortened interval between two reversals of the swimming direction of the cell, the attractant or repellent response. Stimuli are integrated to yield the same response regardless through which photosystem they are given. Simultaneously elicited attractant and repellent signals cancel each other at any time during a reversal interval, even in the period of refractoriness shortly after a reversal, when the cell is insensitive to repellent stimuli. Successively applied stimuli are less completely integrated. The net response depends on the moment of stimulation during the interval, on the sequence of stimuli, and on the delay between them. Integration of successively applied effective stimuli (after refractoriness) is to a great extent explained in terms of a cellular oscillator (A. Schimz and E. Hildebrand, Nature [London] 317:641-643, 1985) which is changed in opposite directions by attractant and repellent signals. Some conclusions on the shape of the oscillator after its disturbance by a stimulus can be made. Integration of signals during refractoriness leads us to postulate an additional step before the oscillator in the sensory pathway. Cancelling of simultaneous opposite signals is thought to proceed at this integrator. It also takes part in the integration of successively evoked signals. At this step signals rapidly decline within 10 ms, and the total life time (at least of repellent signals) does not exceed 1.2 s.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam M., Oesterhelt D. Morphology, function and isolation of halobacterial flagella. J Mol Biol. 1984 Jul 15;176(4):459–475. doi: 10.1016/0022-2836(84)90172-4. [DOI] [PubMed] [Google Scholar]
- Dencher N. A., Hildebrand E. Sensory transduction in Halobacterium halobium: retinal protein pigment controls UV-induced behavioral response. Z Naturforsch C. 1979 Sep-Oct;34(9-10):841–847. doi: 10.1515/znc-1979-9-1030. [DOI] [PubMed] [Google Scholar]
- Hildebrand E., Dencher N. Two photosystems controlling behavioural responses of Halobacterium halobium. Nature. 1975 Sep 4;257(5521):46–48. doi: 10.1038/257046a0. [DOI] [PubMed] [Google Scholar]
- Schimz A., Hildebrand E. Entrainment and temperature dependence of the response oscillator in Halobacterium halobium. J Bacteriol. 1986 May;166(2):689–692. doi: 10.1128/jb.166.2.689-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling W., Schimz A. Photosensory retinal pigments in Halobacterium halobium. Biophys Struct Mech. 1980;6(2):165–169. doi: 10.1007/BF00535752. [DOI] [PubMed] [Google Scholar]
- Spudich E. N., Spudich J. L. Control of transmembrane ion fluxes to select halorhodopsin-deficient and other energy-transduction mutants of Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4308–4312. doi: 10.1073/pnas.79.14.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Mechanism of colour discrimination by a bacterial sensory rhodopsin. Nature. 1984 Dec 6;312(5994):509–513. doi: 10.1038/312509a0. [DOI] [PMC free article] [PubMed] [Google Scholar]


