Abstract
Mechanisms that protect against asthma remain poorly understood. S-nitrosoglutathione (GSNO), an endogenous bronchodilator, is depleted from asthmatic airways, suggesting a protective role. We report that, following allergen challenge, wild-type mice exhibiting airway hyperresponsivity have increased airway levels of the enzyme GSNO reductase (GSNOR) and are depleted of lung S-nitrosothiols (SNOs). In contrast, mice with genetic deletion of GSNOR exhibit increases in lung SNOs and are protected from airway hyperresponsivity. Our results indicate that endogenous SNOs, governed by GSNOR, are critical regulators of airway responsivity and may provide new therapeutic approaches to asthma.
The current understanding of allergic asthma, characterized by airway hyperresponsivity (AHR) and chronic airway inflammation, is centered on the role of bronchoconstrictor substances and inflammatory mediators (1–3). The role of endogenous airway relaxants in the pathogenesis of asthma has received less attention, and the relative importance of impaired airway relaxation versus active constriction is unknown.
Nitric oxide (NO) has been implicated in the regulation of airway tone (4), and elevated levels of NO in the exhaled breath are a signature of asthma (5, 6), attributed in part to up-regulation of cytokine-inducible NO synthase (iNOS) within the airways (7–10). However, neither genetic deletion of iNOS in mice nor pharmacological inhibition of NOS in asthmatic patients has provided significant protection against AHR (7, 10, 11). Furthermore, animals deficient in NO synthases that are expressed constitutively in the lung (eNOS, nNOS, and iNOS) do not exhibit increases in airway tone or AHR (7).
Accumulating evidence indicates that NO bioactivity is conveyed largely through the covalent modification of cysteine sulfurs by NO to form S-nitrosothiols (SNOs) (12–14). Of these, S-nitrosoglutathione (GSNO) represents a major source of bronchodilatory NO bioactivity (airway concentrations of GSNO are much higher than those of NO) (15), and abnormal metabolism of GSNO (reflecting altered NOS activity) has been described in several lung diseases (15–17). Paradoxically, although NOS activity is increased in human asthma, it has been reported that GSNO is depleted from airway lining fluid (ALF) (18, 19); the functional consequences of this disequilibrium are not currently understood. We have recently described an enzyme, GSNO reductase (GSNOR), which governs levels of GSNO [and, indirectly, protein SNO (20)] and which is expressed widely across tissues, including the lung (12, 20). We used mice with targeted deletion of the GSNOR gene (GSNOR−/−) (21) to elucidate the role of SNOs in the regulation of airway tone and pathogenesis of asthma.
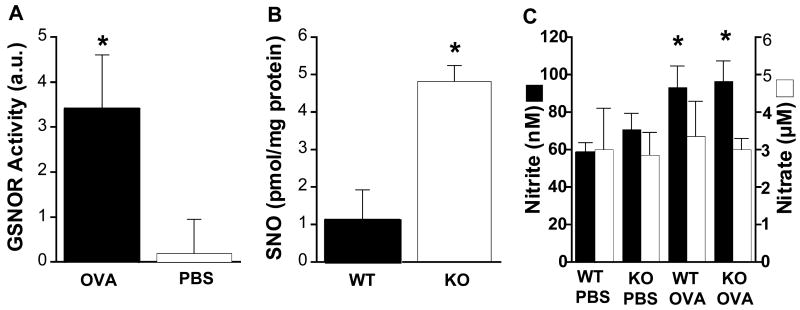
GSNOR activity was first measured in ALF. In wild-type (WT) mice, GSNOR activity was absent from ALF at basal conditions [after phosphate-buffered saline (PBS) treatment] but was readily detected after exposure to the allergen ovalbumin (OVA) (21) (Fig. 1A). As expected, GSNOR activity was not detected in ALF from GSNOR−/− mice under either condition (22). Immunohistochemistry revealed that GSNOR was present in multiple cell types in control and OVA-challenged WT lungs, including airway epithelial cells and infiltrating leukocytes (fig. S1). The presence of GSNOR in ALF after OVA challenge may thus reflect release as a consequence of epithelial damage or lysis of inflammatory cells, as described in asthmatic patients (3, 8).
Fig. 1.
(A) GSNOR activity in bronchoalveolar ALF (cell-free) of WT mice is increased in response to OVA sensitization and challenge. Data represent the mean + SE of samples from at least seven OVA-treated and control (PBS) mice (*, P < 0.05; Student’s two-tailed t test). (B) Levels of S-nitrosylated proteins (SNO) in lung homogenates of GSNOR−/− mice [assayed as described in (12)] were significantly higher than in WT controls after OVA treatment. Data were normalized for protein content of total lysates and represent the mean + SE of at least three WT and GSNOR−/− (KO) mice (*, P < 0.02; Student’s two-tailed t test). (C) Levels of ALF nitrite (filled) and nitrate (open) were similar in WT and GSNOR−/− mice after either control (PBS) or allergen (OVA) treatment. An asterisk indicates significant pairwise differences between OVA-treated and control mice (P < 0.04, n = 12 to 20). Means + SE are shown.
SNO levels were next assayed in homogenates of lung tissue from GSNOR−/− and WT mice. Under basal conditions (PBS treatment), SNO proteins could be detected but only at the limits of sensitivity of the assay used (22, 23). However, after OVA treatment SNO levels were easily quantified (12, 21) and were substantially elevated in GSNOR−/− as compared with WT lungs (Fig. 1B). These results indicate that endogenous SNOs are metabolized by GSNOR under asthmatic-like conditions.
To determine whether the effects of GSNOR deletion were independent of NO generation, levels of nitrite and nitrate (i.e., NO metabolites) were measured in ALF after OVA or PBS treatment. In both GSNOR−/− and WT mice, nitrite concentrations increased after exposure to OVA, whereas nitrate concentrations were unaffected (Fig. 1C). In contrast to SNOs, however, nitrite and nitrate concentrations did not differ significantly in GSNOR−/− versus WT mice (Fig. 1C). In addition, Western blot analysis of lung lysates showed that iNOS expression was comparable in GSNOR−/− and WT mice, both at baseline and after OVA challenge (22). Taken together, these data indicate that the dysregulation of SNO homeostasis in the lungs of GSNOR−/− mice was independent of NOS activity.
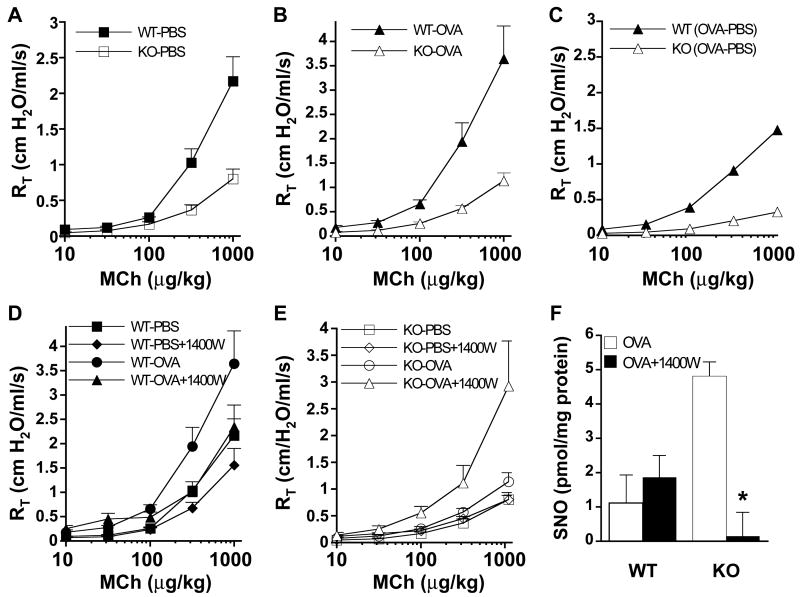
The influence of GSNOR in the regulation of airway tone and development of AHR was next assessed with a Flexivent system in a standard model of allergen-induced asthma (21) (Fig. 2). At baseline (PBS treatment), total pulmonary resistance (RT), which provides a measure of airway tone, was significantly lower in GSNOR−/− than in WT mice (1.5 versus 1.8 cm H2O/ml/s; n = 14 per group, P =0.001), and the airway response to the bron-choconstrictor agonist methacholine (MCh) was significantly lower in PBS-treated GSNOR−/− mice versus WT controls (Fig. 2A; P < 0.001). At high MCh doses, the MCh-induced airway resistance was approximately two-thirds lower in GSNOR−/− than in WT mice (Fig. 2A). RT did not differ at baseline (i.e., premethacholine) after OVA treatment [n = 16 per group (22)]. More important, whereas OVA treatment in WT mice resulted in a marked increase in airway responsiveness (i.e., AHR), OVA-treated GSNOR−/− mice showed little increase in airway responsivity (Fig. 2, B and C; P < 0.004 versus WT), demonstrating that GSNOR−/− mice are protected in this asthma model. These data indicate that GSNOR regulates basal airway tone, as well as airway responsiveness to both bronchoconstrictor agonists and allergen challenge.
Fig. 2.
Airways of GSNOR−/− mice are hyporeactive to methacholine (MCh) and allergen challenge. Total pulmonary resistances (RT) of WT and GSNOR−/− (KO) mice after control (nonallergic; PBS) (A) and allergen (OVA) (B) treatment were determined in the absence or presence of various concentrations of MCh administered intravenously. RT values in PBS-treated and in OVA-treated GSNOR−/− mice were significantly lower than in WT controls [KO PBS versus WT PBS, P < 0.001; KO OVA versus WT OVA, P < 0.004; analysis of variance (ANOVA) and post-hoc analyses at 3- to 5-MCh doses]. Data represent the mean + SE of at least 7 to 10 mice per group. (C) The incremental effect of OVA (over PBS control) on WT and GSNOR−/− mice [OVA minus PBS (OVA-PBS)]. Whereas RT of WT mice increased significantly after OVA treatment (WT PBS versus WT OVA, P < 0.04; ANOVA), the RT of GSNOR −/− mice did not change significantly (KO PBS versus KO OVA, P = 0.1; ANOVA). (D) Effect of the iNOS inhibitor 1400W on airway responsiveness in PBS- and OVA-treated WT mice (WT PBS versus WT PBS + 1400W, P = 0.12, n = 5 to 9; WT OVA versus WT OVA + 1400W, P = 0.22, n = 7 to 9). (E) Effect of iNOS inhibition by 1400W on airway responsiveness in GSNOR−/− mice. Administration of 1400W to OVA-treated GSNOR−/− mice resulted in a significant increase in airway resistance (KO OVA versus KO OVA + 1400W, P < 0.02, n = 5 to 9; ANOVA). (F) Protein S-nitrosylation (SNO) in lung homogenates of OVA-treated mice. iNOS inhibition (1400W) reduces SNO levels in GSNOR−/− mice (*, P < 0.05, n = 3).
To further verify the role of GSNO in the control of airway reactivity and to discriminate its effects from those of NO, WT and GSNOR−/− animals were treated with 1400W, a selective iNOS inhibitor (12). In OVA-treated WT mice, inhibition of iNOS did not significantly alter airway responsiveness (Fig. 2D). In contrast, OVA-treated GSNOR−/− mice displayed markedly heightened airway responsivity after iNOS inhibition (Fig. 2E; P < 0.02), accompanied by a commensurate reduction in SNO levels (Fig. 2F; P < 0.05). Indeed, after iNOS inhibition, neither the levels of SNO nor airway responsivity differed between OVA-treated GSNOR−/− and WT mice (Fig. 2, D, E, and F). iNOS inhibition (1400W) had little effect, however, on the airway responses of PBS-treated control animals (e.g., MCh response in PBS-treated GSNOR−/− mice; Fig. 2E), suggesting that basal airway tone is regulated by eNOS or nNOS. Collectively, these findings indicate that GSNOR−/− mice are protected from allergen challenge and that GSNO activity, originating from iNOS, serves to promote bronchodilation under asthmatic-like conditions. In addition, the genetic evidence suggests a role for GSNO, derived from eNOS or nNOS, in the regulation of basal airway tone.
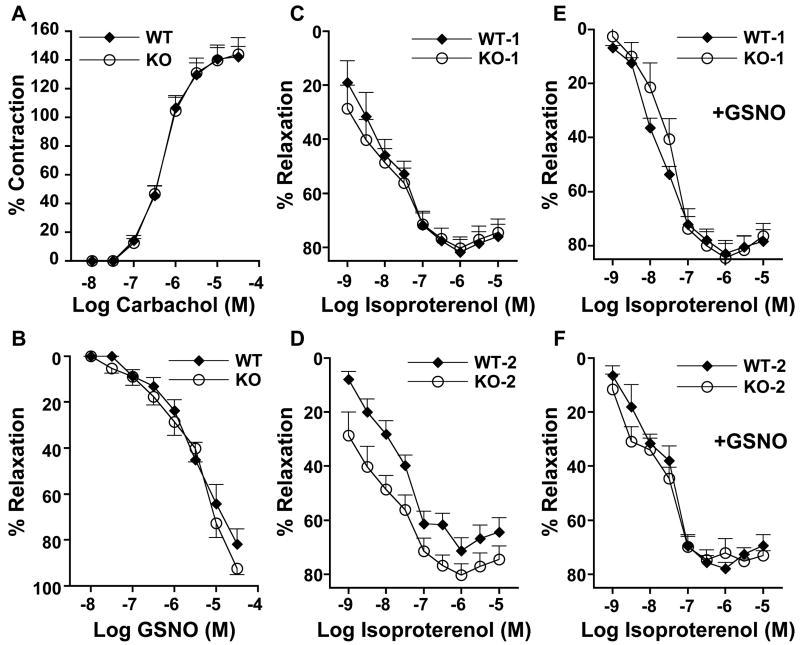
To investigate potential cellular mechanisms by which GSNO regulates airway reactivity, we performed a bioassay (21) that measured the tone of tracheal rings obtained from WT and GSNOR−/− mice (Fig. 3). Tracheal rings from GSNOR−/− and WT mice contracted similarly when stimulated by carbachol, a parasympathetic agonist (Fig. 3A), and relaxed similarly to GSNO (Fig. 3B). Thus, the decreased airway response to MCh in GSNOR−/− mice in vivo (Fig. 2) is unlikely to have resulted from direct effects on cholinergic receptor–mediated activity. In contrast, tracheal relaxation in response to the β-adrenergic agonist, isoproterenol, differed between GSNOR−/− and WT mice. Specifically, tracheal rings of WT mice desensitized upon repeated exposure to isoproterenol, whereas relaxations of tracheal rings from GSNOR−/− mice were maintained (Fig. 3, C and D). Furthermore, the desensitization of WT tracheal rings was prevented by pretreatment with GSNO (Fig. 3, E and F). Thus, abundance of GSNO evidently represents an important determinant of airway responsivity to β-adrenergic agonists. Interestingly, a recent study demonstrated that vascular tachyphylaxis induced by isoproterenol when NOS is inhibited can be prevented by exogenous S-nitrosothiols (24). Taken together, these data suggest that GSNO levels, governed by the catalytic activity of GSNOR, can modulate β-adrenergic responsivity, and that the loss of SNO in asthmatic animals contributes to airway tachyphylaxis.
Fig. 3.
In vitro contractile and relaxant responses of tracheal rings from WT and GSNOR−/− (KO) mice. Rings were contracted by carbachol (A) and relaxed by GSNO (B) or isoproterenol (C to F). Data points are means + SE for n = 6 to 8 in all experiments. In (B) to (F), tracheal rings were precontracted with the EC50 concentration of carbachol [the concentration in (A) producing 50% of maximal contraction]. Relaxation of tracheal rings in response to the first (WT1 and KO1) (C) and second (WT2 and KO2) (D) exposures to isoproterenol. With repeated exposure to isoproterenol, WT rings (WT2) demonstrated a decremental response to β2-stimulation (P < 0.03, WT1 versus WT2; ANOVA), whereas GSNOR−/− rings (KO2) retained their responsivity (P = not significant, KO1 versus KO2). (E and F) Tracheal rings were incubated with 1 μM GSNO for 30 min before exposure to isoproterenol. GSNO-supplemented WT rings show a phenotype indistinguishable from KO (i.e., WT rings no longer desensitize) [(F) versus (D)].
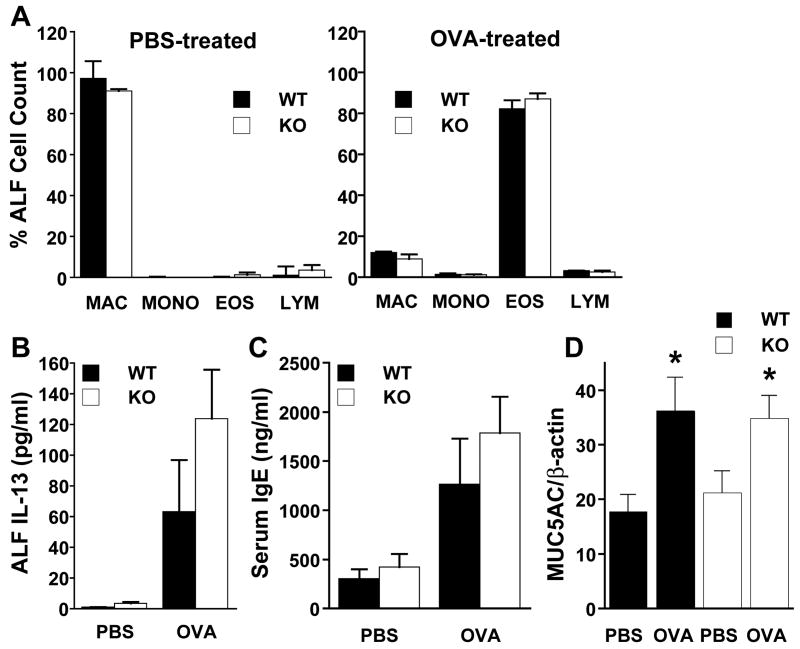
It is generally believed that airway inflammation is central to the pathogenesis of allergic asthma (1, 2). C57BL/6 mice exhibit robust inflammatory responses (fig. S1), which rank highly compared with those of other strains (25). It is therefore noteworthy that the total number and composition of leukocytes, including eosinophils, were indistinguishable in ALF from GSNOR−/− and WT mice after PBS or OVA treatment (Fig. 4A) (21). Furthermore, interleukin-13 (IL-13) (Fig. 4B) and total serum immunoglobulin E (IgE) (Fig. 4C) were closely comparable in GSNOR−/− and WT mice at baseline and increased equivalently after OVA challenge. In addition, the degree of mucus metaplasia, as determined by periodic acid-Schiff staining (21) and mucin gene (MUC5AC) expression (Fig. 4D) were comparable in OVA-treated GSNOR−/− and WT mice. Thus, the protection from asthma in GSNOR−/− mice does not reflect a suppressed response to allergen and importantly reveals that SNOs can preserve airway patency in the face of inflammation.
Fig. 4.
The inflammatory response to asthmatic challenge was not reduced in GSNOR−/− mice. (A) Leukocyte cell differentials (MAC, macrophage; MONO, monocyte; EOS eosinophil; LYM, lymphocyte), (B) IL-13 levels in ALF, and (C) total serum IgE level were determined for WT (filled) and GSNOR−/− (open) mice that had undergone either control (non-allergic; PBS) or allergic (OVA) exposure. Data are means + SE for n = 12 per group. (D) MUC5AC mRNA levels in whole lung (expressed as a ratio of β-actin mRNA levels) were determined for control (nonallergic; PBS) and allergic (OVA) WT and GSNOR−/− (KO) mice. MUC5AC mRNA expression increases comparably in both WT and GSNOR−/− mice after OVA challenge. Values are means + SE, n = 6 in each group. *, P < 0.05, WT PBS versus WT OVA and KO PBS versus KO OVA.
Our findings indicate that the enzyme GSNOR is critical for regulation of airway tone under basal conditions and in response to allergic challenge, and that dysregulation of SNO homeostasis may contribute fundamentally to the asthmatic phenotype. Thus far, the role of NO (and SNO) in asthma has remained a matter of debate. Airway NO is increased (5, 6), but SNOs may be decreased (18, 19) and it has been unclear if NOS activity counters or aggravates airway constriction. We have shown that salutary NO bioactivity in the airways is conveyed largely by SNOs, and in particular, that GSNO protects against AHR. Thus, our results suggest that increases in NO are in fact beneficial when they are channeled adequately into SNOs, and that the key determinant of whether NOS activity exacerbates or protects against asthma may reflect the extent to which SNO-based signaling is preserved (fig. S2). Accordingly, although NOS inhibition is a considered strategy to ameliorate AHR [note trend toward lower AHR in Fig. 2D, consistent with (26)], this approach may deleteriously deplete SNOs and thereby exacerbate asthma (Fig. 2, E and F). Our results further suggest that an explanation for the SNO deficit in asthma (18, 19) may be an increase in SNO turnover resulting from increased GSNOR activity and may thus provide a basis for novel therapeutic strategies to alleviate airway obstruction.
Supplementary Material
References and Notes
- 1.Wills-Karp M, et al. Science. 1998;282:2258. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 2.Grunig G, et al. Science. 1998;282:2261. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn L, Elias JA, Chupp GL. Annu Rev Immunol. 2004;22:789. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 4.Gaston B, Drazen JM, Loscalzo J, Stamler JS. Am J Respir Crit Care Med. 1994;149:538. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- 5.Kharitonov SA, et al. Lancet. 1994;343:133. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 6.Alving K, Weitzberg E, Lundberg JM. Eur Respir J. 1993;6:1368. [PubMed] [Google Scholar]
- 7.De Sanctis GT, et al. J Exp Med. 1999;189:1621. doi: 10.1084/jem.189.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamid Q, et al. Lancet. 1993;342:1510. doi: 10.1016/s0140-6736(05)80083-2. [DOI] [PubMed] [Google Scholar]
- 9.Saleh D, Ernst P, Lim S, Barnes PJ, Giaid A. FASEB J. 1998;12:929. [PubMed] [Google Scholar]
- 10.Hansel TT, et al. FASEB J. 2003;17:1298. doi: 10.1096/fj.02-0633fje. [DOI] [PubMed] [Google Scholar]
- 11.Xiong Y, Karupiah G, Hogan SP, Foster PS, Ramsay AJ. J Immunol. 1999;162:445. [PubMed] [Google Scholar]
- 12.Liu L, et al. Cell. 2004;116:617. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 13.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Nat Rev Mol Cell Biol. 2005;6:150. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 14.Foster MW, McMahon TJ, Stamler JS. Trends Mol Med. 2003;9:160. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 15.Gaston B, et al. Proc Natl Acad Sci USA. 1993;90:10957. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grasemann H, Gaston B, Fang K, Paul K, Ratjen F. J Pediatr. 1999;135:770. doi: 10.1016/s0022-3476(99)70101-0. [DOI] [PubMed] [Google Scholar]
- 17.Snyder AH, et al. Am J Respir Crit Care Med. 2002;165:922. doi: 10.1164/ajrccm.165.7.2105032. [DOI] [PubMed] [Google Scholar]
- 18.Gaston B, et al. Lancet. 1998;351:1317. doi: 10.1016/S0140-6736(97)07485-0. [DOI] [PubMed] [Google Scholar]
- 19.Dweik RA, et al. Proc Natl Acad Sci USA. 2001;98:2622. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, et al. Nature. 2001;410:490. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 21.Materials and methods are available as supporting material on Science Online
- 22.L. G. Que, L. Liu, J. S. Stamler, unpublished data.
- 23.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Nat Cell Biol. 2001;3:193. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 24.Whalen EJ, Johnson AK, Lewis SJ. Hypertension. 2000;36:376. doi: 10.1161/01.hyp.36.3.376. [DOI] [PubMed] [Google Scholar]
- 25.Whitehead GS, Walker JK, Berman KG, Foster WM, Schwartz DA. Am J Physiol Lung Cell Mol Physiol. 2003;285:L32. doi: 10.1152/ajplung.00390.2002. [DOI] [PubMed] [Google Scholar]
- 26.Koarai A, et al. Pulm Pharmacol Ther. 2000;13:267. doi: 10.1006/pupt.2000.0254. [DOI] [PubMed] [Google Scholar]
- 27.This paper has been reviewed and approved for release by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency. Approval does not signify that the contents necessarily reflect the views and policies of the U.S. EPA, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. J.S.S. is a paid consultant to Nitrox LLC, a biotechnology company developing NO-based drugs for disorders of heart, lung, and blood. This work was supported by NIH ES012496 (J.S.S.) and HL004171 (L.G.Q.), and by an award to J.S.S. from the Sandler Program for Asthma Research. We thank N. Coates and W. M. Foster for their assistance.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.