Abstract
Voltage-gated potassium (Kv) channels generate the outward K+ ion currents that constitute the primary force in ventricular repolarization. Kv channels comprise tetramers of pore-forming α subunits and, in probably the majority of cases in vivo, ancillary or β subunits that help define the properties of the Kv current generated. Ancillary subunits can be broadly categorized as cytoplasmic or transmembrane, and can modify Kv channel trafficking, conductance, gating, ion selectivity, regulation and pharmacology. Because of their often profound effects on Kv channel function, studies of the molecular correlates of ventricular repolarization must take into account ancillary subunits as well as α subunits. Cytoplasmic ancillary subunits include the Kvβ subunits, which regulate a range of Kv channels and may link channel gating to redox potential; and the KChIPs, which appear most often associated with Kv4 subfamily channels that generate the ventricular Ito current. Transmembrane ancillary subunits include the MinK-related proteins (MiRPs) encoded by KCNE genes, which modulate members of most Kv α subunit subfamilies; and the putative 12-transmembrane domain KCR1 protein which modulates hERG. In some cases, such as the ventricular IKs channel complex, it is well-established that the KCNQ1 α subunit must co-assemble with the MinK (KCNE1) single transmembrane domain ancillary subunit for recapitulation of the characteristic, unusually slowly-activating IKs current. In other cases it is not so clear-cut, and in particular the roles of the other MinK-related proteins (MiRPs 1–4) in regulating cardiac Kv channels such as KCNQ1 and hERG in vivo are under debate. MiRP1 alters hERG function and pharmacology, and inherited MiRP1 mutations are associated with inherited and acquired arrhythmias, but controversy exists over the native role of MiRP1 in regulating hERG (and therefore ventricular IKr) in vivo. Some ancillary subunits may exhibit varied expression to shape spatial Kv current variation, e.g. KChIP2 and the epicardial-endocardial Ito current density gradient. Indeed, it is likely that most native ventricular Kv channels exhibit temporal and spatial heterogeneity of subunit composition, complicating both modeling of their functional impact on the ventricular action potential and design of specific current-targeted compounds. Here, we discuss current thinking and lines of experimentation aimed at resolving the complexities of the Kv channel complexes that repolarize the human ventricular myocardium.
Background
Cellular repolarization, terminating the action potential, is driven by the efflux of K+ ions through highly K+ ion-selective, voltage-gated potassium (Kv) channels. Kv channels consist of a tetramer of α subunits each with six transmembrane domains, and including a voltage sensor and pore region. The four pore regions contribute to a single, pseudo four-fold symmetrical aqueous pathway for K+ ions in the center of the channel [1, 2]. Following cellular depolarization driven by Na+ influx through voltage-gated sodium channels, each of the Kv channel voltage sensors moves essentially independently but all four must be activated by cellular depolarization for the activation gate to open and permit K+ ion efflux (hence ‘voltage-gated’). The fourth transmembrane helix (S4) bears periodic basic residues and is the primary voltage sensor, with S1–S3 providing charge shielding and probably aiding folding of the voltage-sensing apparatus [3]. A loop between S4 and S5 (the S4–S5 linker) communicates S4 position to the activation gate at the intracellular end of the conduction pathway-lining S6 domain via S5, and the selectivity filter and pore-helix lie between S5 and S6 in the primary structure (Figure 1) [4].
Figure 1.
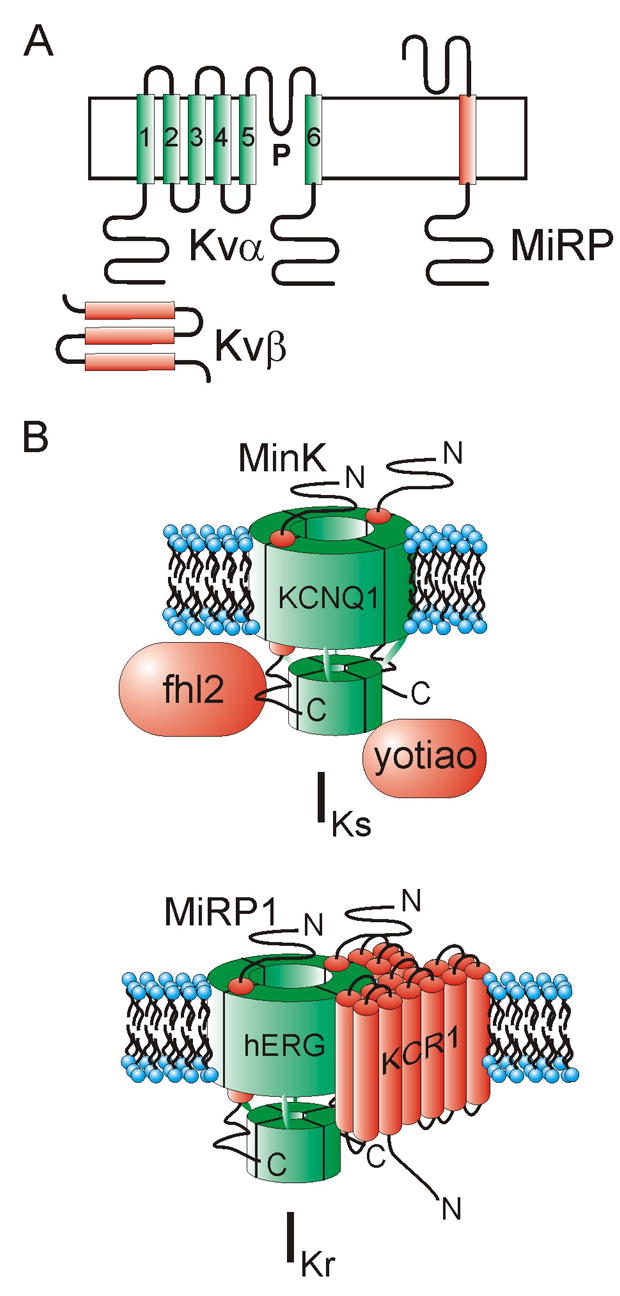
A. Topologies of Kvα, Kvβ and MiRP subunits.
B. Cartoon of IKs (upper) and IKr (lower) complexes with proposed α and ancillary subunits illustrated.
In ventricular myocytes, action potential duration and morphology must be exquisitely maintained to sustain normal cardiac rhythm. Timely repolarization ends the action potential to ensure a corrected electrocardiogram QT interval (QTc, the time between the onset of ventricular depolarization and the end of repolarization, corrected for heart-rate) of below 440 ms in healthy adults. Increased depolarizing force (generally caused by reduced voltage-gated sodium channel inactivation) or decreased repolarizing force (reduced Kv channel current due to genetic, pharmacologic or other factors) increases the QTc and can cause early afterdepolarizations (EADs) which beget torsades de pointes and the potentially lethal ventricular fibrillation [5]. While voltage-gated sodium channel gating kinetics vary little with cell type (even e.g. cardiac myocytes versus rapid-spiking neurons), the gating kinetics of Kv channels can vary between subtypes over several orders of magnitude even within the same cell. Gating kinetics - together with the relative expression levels of each Kv channel type in a particular cell - are a prime determinant of the temporal dimensions of the action potential because they control the relative repolarizing force at specific time-points during the action potential. To understand how adverse genetic and pharmacologic factors cause cardiac arrhythmia by altering repolarizing force and the duration and shape of the ventricular action potential, we must understand the subunits that generate Kv currents and the variety of factors that facilitate and influence subunit assembly and trafficking, and channel regulation.
There are ~40 Kv channel α subunit genes, each encoding a functionally distinct α subunit with the same six-transmembrane topology. Many of these α subunits can form heterotetramers with other α subunits; generally, but perhaps not always, within the same gene subfamily e.g. Kv4.2 with Kv4.3. This increases functional diversity by creating channels often with a phenotype distinct from that of homotetramers of either α subunit. Functional diversity is also increased, and often in a dynamic manner, by alternative splicing and post-translational modifications including phosphorylation [6–8]. The remainder of this review concentrates on a third mechanism of increasing functional repertoire: modification by ancillary subunits. These are proteins generally not absolutely required for construction of a pore, but part and parcel of many, if not all, native channel complexes and essential for recapitulation of specific native current properties. We contend that attaining a greater understanding of the extent and intricacies of α-ancillary subunit interactions should be a major goal for academic and industry ion channel researchers alike. For more comprehensive reviews from this lab on ancillary subunits and their impact on physiology, pathophysiology and pharmacology, see: [9, 10].
What we know
Kv channel ancillary subunits can be broadly categorized into two types: cytoplasmic and transmembrane. The first cloned Kv channel ancillary subunits were the Kvβ subunits. These assemble as a tetramer beneath the α subunits, attached to the cytoplasmic N-termini of the α subunits [11, 12]. Kvβ subunits co-assemble with α subunits from several different subfamilies including Kv1.5, which is important for atrial repolarization but is not thought to be expressed significantly in human ventricular myocytes [13]. This distinction has led to extensive preclinical trials examining the use of Kv1.5 blockers as atrial fibrillation therapies as they preferentially lengthen the atrial myocyte action potential over the ventricular action potential, thus avoiding long QT syndrome (LQTS) [14]. Kvβ subunits can serve several functions: they promote forward trafficking of α subunits, accelerate inactivation by providing an inactivation domain for fast (N-type) inactivation, and can act as a redox sensor, linking channel function to the redox state of the cell interior [15–17]. Kvβ1.2 co-assembles with Kv1.5, accelerating its inactivation in heterologous expression experiments (hereafter referred to as ‘in vitro’) but we do not yet know when and where this occurs in vivo [18].
The other major class of cytoplasmic Kv channel ancillary subunits, the KChIPs, primarily regulate Kv4 family α subunits, which generate the transient outward current (Ito) in mammals including mice and men [19, 20]. KChIPs form a tetramer at the base of the Kv4 α subunit tetramer, interacting with primarily the N-terminus and probably also the C-terminus of the α subunit [21]. KChIPs promote surface expression of α subunits and also slow Kv4 inactivation [20]. Interestingly, KChIP2 exhibits a strong expression gradient in canine and human ventricular wall, with 25-fold greater abundance in canine epicardium than endocardium [22]. This mirrors Ito current expression, probably illustrating a primary role of ancillary subunits in general, that of providing functional diversity against a more uniform α subunit background; in this case by promoting α subunit surface expression, the KChIP2 gradient essentially dictates Ito spatial density and also kinetics. Kv4-KChIP complexes form not only in the heart, but also in the brain, in complex with single transmembrane domain proteins from the DPPX family (not from the KCNE gene family, see below) [23]. KChIP2 knockout mice exhibit a prolonged elevation in the ST segment and are highly susceptible to induction of cardiac arrhythmias due to loss of Ito and increased action potential duration [24], but KChIP mutations have not so far been linked to LQTS in man. This, however, is consistent with the lack of Kv4 gene mutations associating with human LQTS, and probably reflects the relative lack of importance of Ito in human ventricular repolarization.
By far the most studied transmembrane Kv ancillary subunits are the KCNE subunits, also called MinK-related Proteins (MiRPs). These span the membrane once and comprise a 5-member family of promiscuous modifiers of Kv α subunit function [9]. We know that they are essential for ventricular repolarization in some cases because mutations in them are associated with human LQTS. The first-known member, MinK (encoded by KCNE1) associates with the KCNQ1 α subunit in the ventricles of mammals including guinea-pig, horse and man. This co-assembly slows KCNQ1 activation 5–10 fold, increases unitary conductance 4-fold, removes inactivation and alters regulation and pharmacology of the complex. MinK-KCNQ1 channels generate the IKs current which contributes to human ventricular repolarization [25–28].
Homomeric KCNQ1 channels are not thought to generate native currents in human ventricles. KCNE1 mutations that reduce IKs currents are associated with two forms of inherited LQTS: Romano-Ward syndrome and Jervell and Lange-Nielsen syndrome. The latter also presents as sensorineural deafness, because IKs channels also mediate K+ ion secretion into the endolymph of the inner ear [29–32].
The extraordinarily slow activation of IKs channels appears to provide a slowly building repolarizing force that contributes much during phase 3 repolarization. Conventional wisdom has been that IKs does not contribute significantly to ventricular repolarization in mice, which is instead dominated by the much faster activating Ito current. However, in a recent study targeted KCNE1 disruption was shown for the first time to cause ventricular abnormalities including prolonged APD90 and EADs [33]. In man, KCNQ1 mutations are roughly equal to hERG mutations as the most frequently identified mutant genes in inherited LQTS patients in which a mutation has been isolated (40–45 % each). KCNE1 mutations are a rarer cause of LQTS, at <5 % of genotyped cases. However, the KCNE1 gene is one fifth the size of the KCNQ1 gene, so this alone tends to make mutations in it less frequent than in KCNQ1 (assuming a similar rate of mutation per base) [33].
One can probably conclude that MinK, the KCNE1 gene product, plays a substantial role in IKs and is commonly associated with KCNQ1 in vivo in human ventricles. Recent modeling studies suggest that MinK also endows KCNQ1 channels with the capacity to accumulate in closed states that are near to the open state, thus providing a safety-net (repolarization reserve) in case of failure of IKr, which is probably the dominant repolarizing current in human ventricles [34]. However, MiRP promiscuity probably renders the actual situation more complex than this, because among other things MinK can also regulate hERG, and other MiRPs can regulate KCNQ1, with a range of resultant channel phenotypes – at least in vitro (see ‘What we don’t know’) [35–37]. Further, native IKs complexes are formed from a complex of not just KCNQ1 and ancillary subunits such as MinK, but also yotiao, which mediates interaction with cAMP-depndent PKA and protein phosphatase 1, placing IKs complexes under β-adrenergic control [38] (Figure 1 B).
MiRP1 regulates hERG in vitro, accelerating its deactivation 2–3 fold and reducing unitary conductance 40%. Loss-of-function MiRP1 mutations associate with inherited LQTS (~1–2% of positively genotyped cases) [39]. The rather subtle effects of MiRP1 on hERG have led to arguments regarding its role in modulating IKr in human ventricles, but compelling supporting evidence has been provided by identification in drug-induced arrhythmia patients of MiRP1 mutations and polymorphisms that increase sensitivity of MiRP1-hERG channels to block by the LQTS-causing drugs, e.g. T8A polymorphism and sulfamethoxazole; Q9E polymorphism and clarithromycin [39, 40]. In addition, these two cases may argue in the future for genotyping of acquired LQTS susceptibility genes such as KCNE2 (which encodes MiRP1) before prescription of certain drugs – T8A is present in 1.6% of U.S. Caucasians and Q9E in 3% of African-Americans, numbers not to be ignored [40, 41]. IKr complexes may also be regulated in vivo by KCR1, a putative 12-transmembrane domain subunit that alters the influence of MiRP1 on hERG pharmacology [42, 43], further complicating attempts to predict acquired arrhythmia susceptibility (Figure 1 B).
What we don’t know
The greatest dearth of knowledge with respect to ancillary subunits is how much and how many of the plethora of α-ancillary subunit interactions we observe in vitro occur in vivo. This is not simply answered by co-immunoprecipitating two subunits from native ventricle, or even by tissue targeted knockout, because as with KChIP2 there are likely spatial and/or temporal gradients for ancillary subunit expression. Add subunit promiscuity and the spoiler that the functional consequences of many such interactions vary with cell-type, metabolic state and a host of other factors and the possible array of interactions and their ramifications at any given time is massive and also likely to be changing constantly.
KCNQ1, and its interactions with MiRPs, serves as a prime example. MinK-KCNQ1 channels form in the ventricles and the inner ear, with MinK slowing KCNQ1 activation and mutations in either subunit associating with inherited LQTS. MiRP1 (KCNE2) associates in vitro with KCNQ1 to form a constitutively-active channel which is pH-sensitive. Both subunits are expressed in parietal cells of the gut and kcne2 −/− mice exhibit achlorhydria, gastric hyperplasia and hypergastrinemia due to severely impaired parietal cell proton secretion [44–46]. Both subunits are also expressed in human, canine and rat ventricle [47], but whether they co-assemble there is unknown. Studies are currently underway in our laboratory to explore the cardiac phenotype of kcne2 −/− mice. KCNQ1 is also converted to a constitutively active K+ channel by MiRP2 (KCNE3); this complex probably forms in the colon but whether MiRP2 modulates KCNQ1 in ventricular myocytes of any species is unknown [37]. MiRP3 (KCNE4) inhibits KCNQ1 currents [48] and MiRP4 (KCNE5) positively shifts KCNQ1 activation and slows activation, qualitatively similar to the effects of MinK [49]. All five KCNE-encoded subunits are reportedly expressed in the ventricles of various mammalian species including human, but the roles of all five are either partially or wholly unknown [50]. Mutations or polymorphisms in KCNE4 and KCNE5 genes have also been variously associated with LQTS or atrial fibrillation, but these correlations are being treated with caution at present [51–53]. In addition, the possibility of IKs complexes containing more than one type of KCNE subunit in a single macromolecular complex is currently under debate [54], and this together with potential mechanisms for switching partners suggests that a highly diverse and dynamic array of KCNQ1-KCNE complexes may constitute IKs [50].
Reciprocally, each ancillary subunit is potentially involved in an array of interactions. For example, MiRP1 regulates KCNQ1 in parietal cells and, based on drug-induced arrhythmia genetic data and native co-imunoprecipitations, hERG in mammalian heart [39, 46, 55]. According to in vitro data, MiRP1 also modulates pacemaker (HCN), Kv4 subfamily and Kv3 subfamily α subunits, all of which are reportedly expressed in the heart of one or more mammalian model species and/or man [9].
To summarize, Kv channel ancillary subunits play an important and complex role in ventricular repolarization which is at present largely ill-defined. A concerted approach utilizing genetic techniques (human and model systems), improved molecular imaging approaches, and sophisticated spatial-temporal resolution will be required to elucidate the true scope of α-ancillary subunit interactions, the dynamic nature of these interactions and the processes controlling this dynamism, and the evolutionary advantages presumably gained from this elaborate basis for generation of ventricular repolarization currents.
Acknowledgments
G.W.A. is grateful for financial support from the NIH (R01 HL079275; RO3 DC07060).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350(6315):232–5. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- 2.MacKinnon R. Potassium channels and the atomic basis of selective ion conduction (Nobel Lecture) Angew Chem Int Ed Engl. 2004;43(33):4265–77. doi: 10.1002/anie.200400662. [DOI] [PubMed] [Google Scholar]
- 3.Papazian DM, et al. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature. 1991;349(6307):305–10. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- 4.Shieh CC, Klemic KG, Kirsch GE. Role of transmembrane segment S5 on gating of voltage-dependent K+ channels. J Gen Physiol. 1997;109(6):767–78. doi: 10.1085/jgp.109.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackerman MJ. The long QT syndrome: ion channel diseases of the heart. Mayo Clin Proc. 1998;73(3):250–69. doi: 10.4065/73.3.250. [DOI] [PubMed] [Google Scholar]
- 6.Kim M, et al. Alternative splicing in the pore-forming region of shaker potassium channels. J Neurosci. 1997;17(21):8213–24. doi: 10.1523/JNEUROSCI.17-21-08213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang GH, et al. Molecular analyses of KCNQ1-5 potassium channel mRNAs in rat and guinea pig inner ears: expression, cloning, and alternative splicing. Acta Otolaryngol. 2006;126(4):346–52. doi: 10.1080/00016480500416777. [DOI] [PubMed] [Google Scholar]
- 8.Abbott GW, Butler MH, Goldstein SA. Phosphorylation and protonation of neighboring MiRP2 sites: function and pathophysiology of MiRP2-Kv3.4 potassium channels in periodic paralysis. Faseb J. 2006;20(2):293–301. doi: 10.1096/fj.05-5070com. [DOI] [PubMed] [Google Scholar]
- 9.McCrossan ZA, Abbott GW. The MinK-Related Peptides. Neuropharmacology. 2004;47(6):787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Panaghie G, Abbott GW. The impact of ancillary subunits on small-molecule interactions with voltage-gated potassium channels. Curr Pharm Des. 2006;12(18):2285–302. doi: 10.2174/138161206777585175. [DOI] [PubMed] [Google Scholar]
- 11.Scott VE, et al. Primary structure of a beta subunit of alpha-dendrotoxin-sensitive K+ channels from bovine brain. Proc Natl Acad Sci U S A. 1994;91(5):1637–41. doi: 10.1073/pnas.91.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulbis JM, et al. Structure of the cytoplasmic beta subunit-T1 assembly of voltage-dependent K+ channels. Science. 2000;289(5476):123–7. doi: 10.1126/science.289.5476.123. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73(6):1061–76. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- 14.Brendel J, Peukert S. Blockers of the Kv1.5 channel for the treatment of atrial arrhythmias. Curr Med Chem Cardiovasc Hematol Agents. 2003;1(3):273–87. doi: 10.2174/1568016033477441. [DOI] [PubMed] [Google Scholar]
- 15.Majumder K, et al. Molecular cloning and functional expression of a novel potassium channel beta-subunit from human atrium. FEBS Lett. 1995;361(1):13–6. doi: 10.1016/0014-5793(95)00120-x. [DOI] [PubMed] [Google Scholar]
- 16.Gulbis JM. The beta subunit of Kv1 channels: voltage-gated enzyme or safety switch? Novartis Found Symp. 2002;245:127–41. discussion 141–5, 165–8. [PubMed] [Google Scholar]
- 17.Weng J, et al. Modulation of voltage-dependent Shaker family potassium channels by an aldo-keto reductase. J Biol Chem. 2006;281(22):15194–200. doi: 10.1074/jbc.M513809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Biasi M, et al. Open channel block of human heart hKv1.5 by the beta-subunit hKv beta 1.2. Am J Physiol. 1997;272(6 Pt 2):H2932–41. doi: 10.1152/ajpheart.1997.272.6.H2932. [DOI] [PubMed] [Google Scholar]
- 19.Blair TA, et al. Functional characterization of RK5, a voltage-gated K+ channel cloned from the rat cardiovascular system. FEBS Lett. 1991;295(1–3):211–3. doi: 10.1016/0014-5793(91)81420-d. [DOI] [PubMed] [Google Scholar]
- 20.An WF, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403(6769):553–6. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 21.Kim LA, et al. Three-dimensional structure of I(to); Kv4.2-KChIP2 ion channels by electron microscopy at 21 Angstrom resolution. Neuron. 2004;41(4):513–9. doi: 10.1016/s0896-6273(04)00050-9. [DOI] [PubMed] [Google Scholar]
- 22.Rosati B, et al. Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. J Physiol. 2001;533(Pt 1):119–25. doi: 10.1111/j.1469-7793.2001.0119b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadal MS, et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37(3):449–61. doi: 10.1016/s0896-6273(02)01185-6. [DOI] [PubMed] [Google Scholar]
- 24.Kuo HC, et al. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell. 2001;107(6):801–13. doi: 10.1016/s0092-8674(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 25.Finley MR, et al. Expression and coassociation of ERG1, KCNQ1, and KCNE1 potassium channel proteins in horse heart. Am J Physiol Heart Circ Physiol. 2002;283(1):H126–38. doi: 10.1152/ajpheart.00622.2001. [DOI] [PubMed] [Google Scholar]
- 26.Sanguinetti MC, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384(6604):80–3. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 27.Barhanin J, et al. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384(6604):78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 28.Sesti F, Goldstein SA. Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome. J Gen Physiol. 1998;112(6):651–63. doi: 10.1085/jgp.112.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Splawski I, et al. Molecular basis of the long-QT syndrome associated with deafness. N Engl J Med. 1997;336(22):1562–7. doi: 10.1056/NEJM199705293362204. [DOI] [PubMed] [Google Scholar]
- 30.Splawski I, et al. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17(3):338–40. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 31.Neyroud N, et al. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet. 1997;15(2):186–9. doi: 10.1038/ng0297-186. [DOI] [PubMed] [Google Scholar]
- 32.Tyson J, et al. IsK and KvLQT1: mutation in either of the two subunits of the slow component of the delayed rectifier potassium channel can cause Jervell and Lange-Nielsen syndrome. Hum Mol Genet. 1997;6(12):2179–85. doi: 10.1093/hmg/6.12.2179. [DOI] [PubMed] [Google Scholar]
- 33.Thomas G, et al. Mechanisms of ventricular arrhythmogenesis in mice following targeted disruption of KCNE1 modelling long QT syndrome 5. J Physiol. 2007;578(Pt 1):99–114. doi: 10.1113/jphysiol.2006.118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva J, Rudy Y. Subunit interaction determines IKs participation in cardiac repolarization and repolarization reserve. Circulation. 2005;112(10):1384–91. doi: 10.1161/CIRCULATIONAHA.105.543306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald TV, et al. A minK-HERG complex regulates the cardiac potassium current I(Kr) Nature. 1997;388(6639):289–92. doi: 10.1038/40882. [DOI] [PubMed] [Google Scholar]
- 36.Tinel N, et al. KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. Embo J. 2000;19(23):6326–30. doi: 10.1093/emboj/19.23.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroeder BC, et al. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403(6766):196–9. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- 38.Marx SO, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295(5554):496–9. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 39.Abbott GW, et al. MiRP1 Forms IKr Potassium Channels with HERG and Is Associated with Cardiac Arrhythmia. Cell. 1999;97(2):175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 40.Sesti F, et al. A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proc Natl Acad Sci U S A. 2000;97(19):10613–8. doi: 10.1073/pnas.180223197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ackerman MJ, et al. Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clin Proc. 2003;78(12):1479–87. doi: 10.4065/78.12.1479. [DOI] [PubMed] [Google Scholar]
- 42.Kupershmidt S, et al. The IKr drug response is modulated by KCR1 in transfected cardiac and noncardiac cell lines. Faseb J. 2003;17(15):2263–5. doi: 10.1096/fj.02-1057fje. [DOI] [PubMed] [Google Scholar]
- 43.Nakajima T, et al. HERG is protected from pharmacological blockade by constitutive alpha -1,2-glucosyltransferase function. J Biol Chem. 2006 doi: 10.1074/jbc.M605976200. [DOI] [PubMed] [Google Scholar]
- 44.Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Arch. 2001;442(6):896–902. doi: 10.1007/s004240100609. [DOI] [PubMed] [Google Scholar]
- 45.Grahammer F, et al. The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology. 2001;120(6):1363–71. doi: 10.1053/gast.2001.24053. [DOI] [PubMed] [Google Scholar]
- 46.Roepke TK, et al. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem. 2006 doi: 10.1074/jbc.M604155200. [DOI] [PubMed] [Google Scholar]
- 47.Jiang M, et al. KCNE2 protein is expressed in ventricles of different species, and changes in its expression contribute to electrical remodeling in diseased hearts. Circulation. 2004;109(14):1783–8. doi: 10.1161/01.CIR.0000124225.43852.50. [DOI] [PubMed] [Google Scholar]
- 48.Grunnet M, et al. KCNE4 is an inhibitory subunit to the KCNQ1 channel. J Physiol. 2002;542(Pt 1):119–30. doi: 10.1113/jphysiol.2002.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Angelo K, et al. KCNE5 Induces Time- and Voltage-Dependent Modulation of the KCNQ1 Current. Biophys J. 2002;83(4):1997–2006. doi: 10.1016/S0006-3495(02)73961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lundquist AL, et al. Expression of multiple KCNE genes in human heart may enable variable modulation of I(Ks) J Mol Cell Cardiol. 2005;38(2):277–87. doi: 10.1016/j.yjmcc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Hofman-Bang J, et al. Does KCNE5 play a role in long QT syndrome? Clin Chim Acta. 2004;345(1–2):49–53. doi: 10.1016/j.cccn.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 52.Ravn LS, et al. Relation of 97T polymorphism in KCNE5 to risk of atrial fibrillation. Am J Cardiol. 2005;96(3):405–7. doi: 10.1016/j.amjcard.2005.03.086. [DOI] [PubMed] [Google Scholar]
- 53.Zeng Z, et al. The Single Nucleotide Polymorphisms of I(Ks) Potassium Channel Genes and Their Association with Atrial Fibrillation in a Chinese Population. Cardiology. 2006;108(2):97–103. doi: 10.1159/000095943. [DOI] [PubMed] [Google Scholar]
- 54.Wu DM, et al. KCNE2 is colocalized with KCNQ1 and KCNE1 in cardiac myocytes and may function as a negative modulator of I(Ks) current amplitude in the heart. Heart Rhythm. 2006;3(12):1469–80. doi: 10.1016/j.hrthm.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 55.Anantharam A, Abbott GW. Does hERG coassemble with a beta subunit? Evidence for roles of MinK and MiRP1. Novartis Found Symp. 2005;266:100–12. discussion 112–7, 155–8. [PubMed] [Google Scholar]


