Abstract
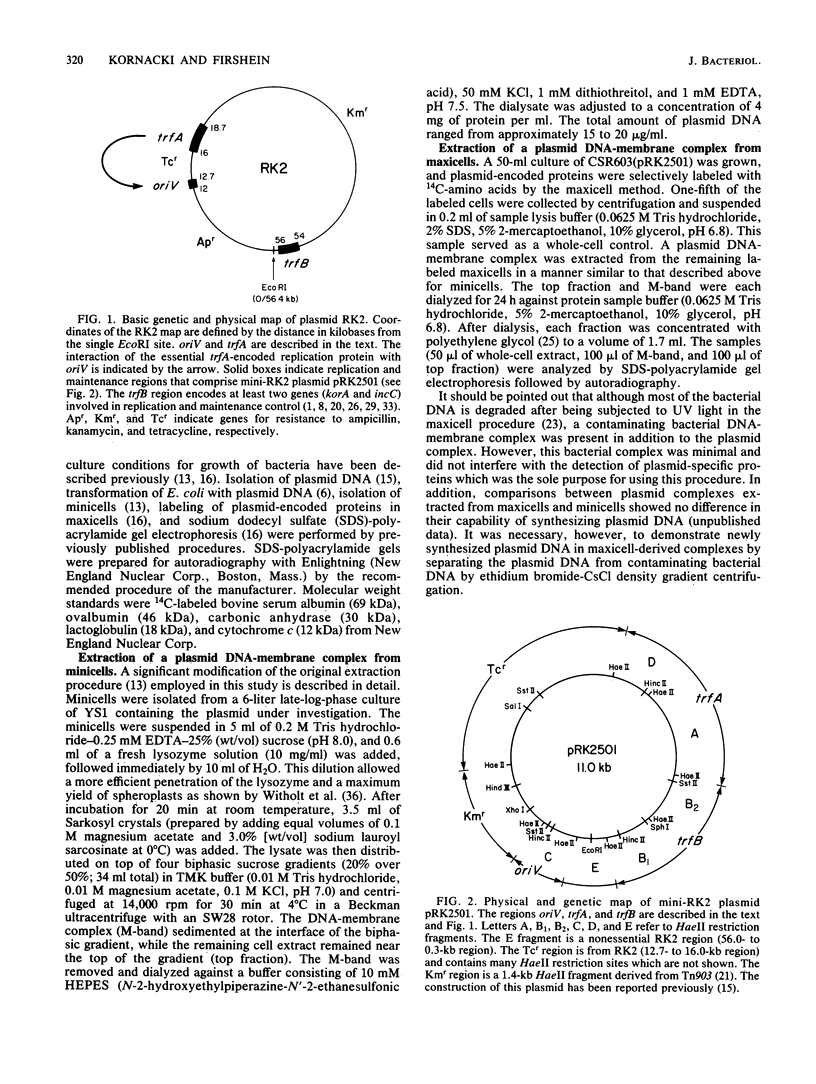
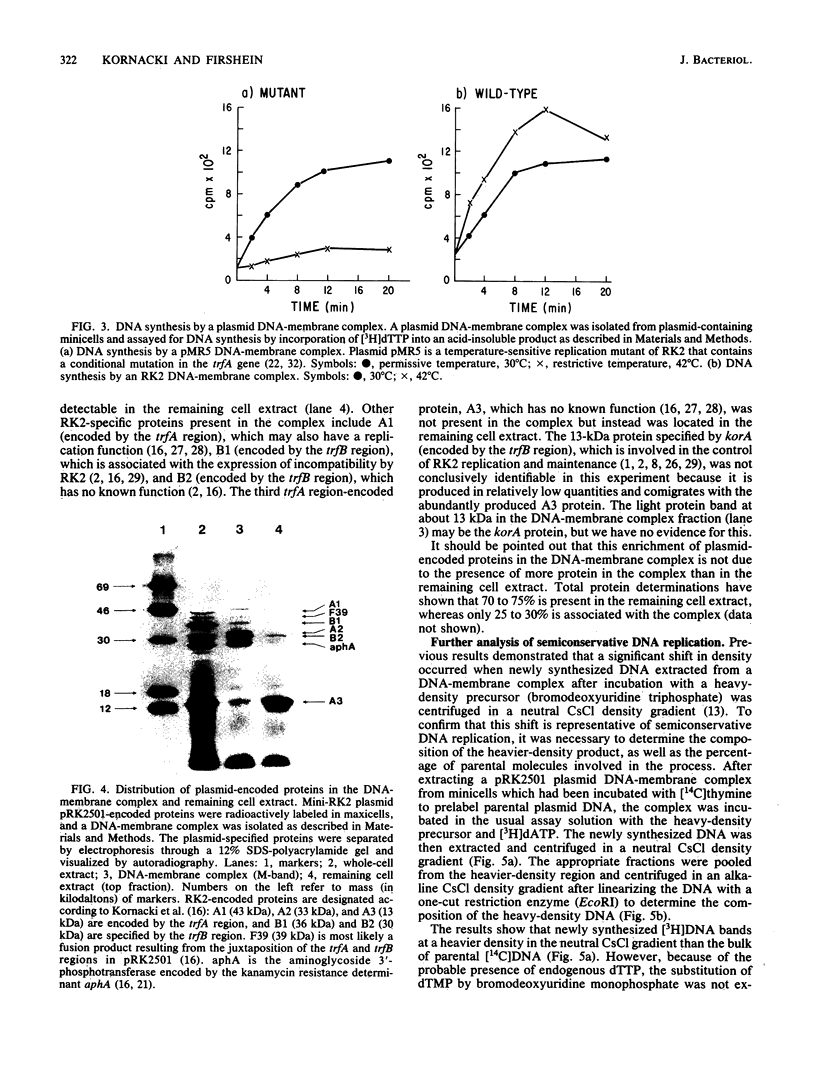
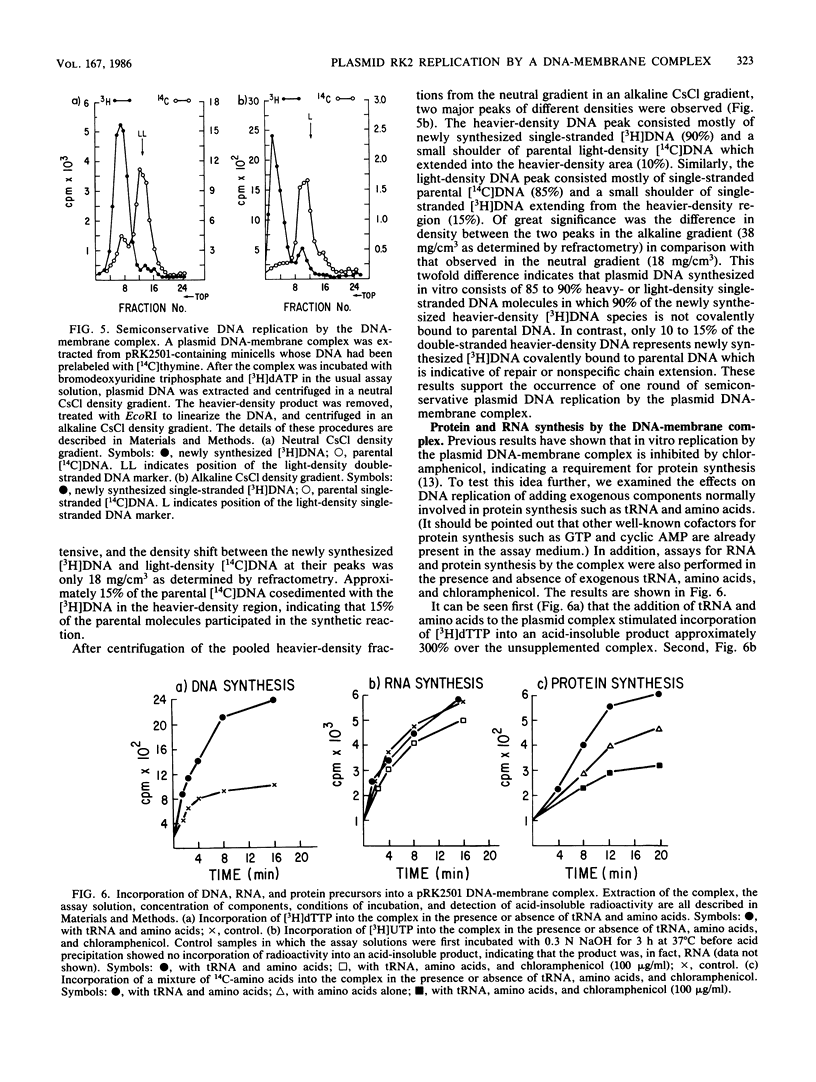
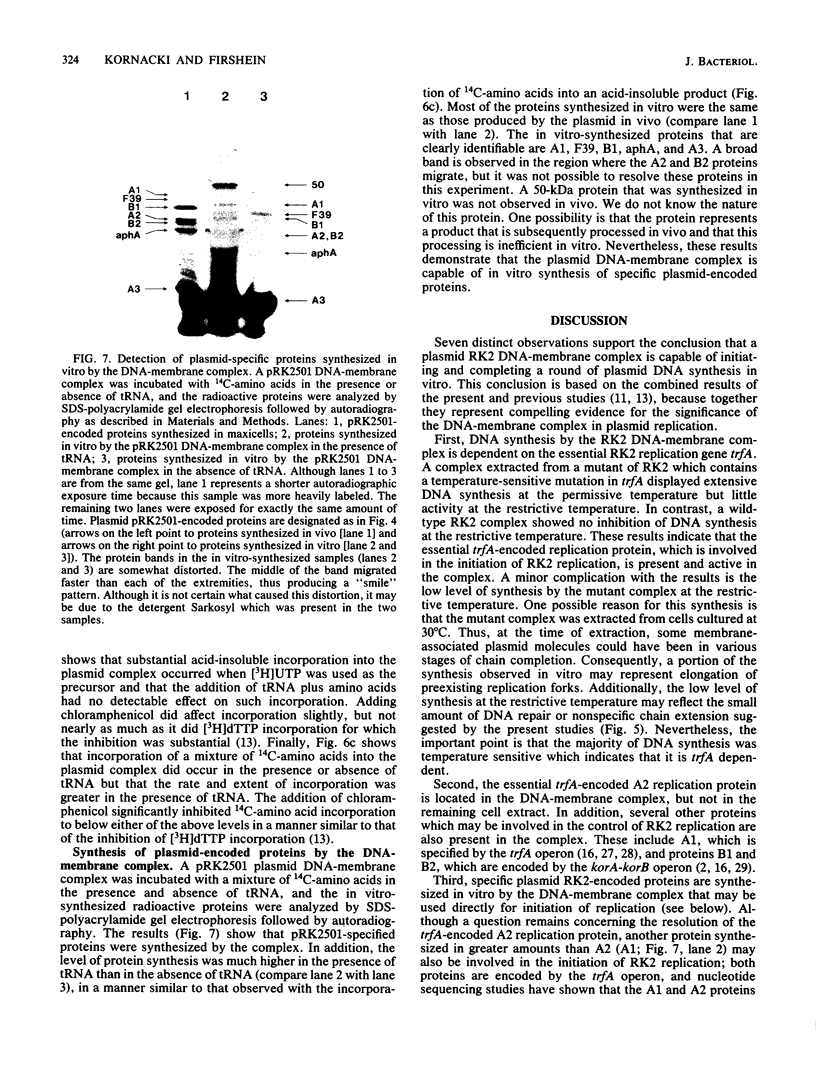
The following results with an in vitro replication system utilizing a plasmid RK2 DNA-membrane complex indicate that the essential trfA-encoded replication protein of RK2 is present and active in the complex. (i) A complex extracted from a conditional replication mutant of RK2, which contains a temperature-sensitive mutation in trfA, displayed extensive DNA synthesis at the permissive temperature but little activity at the restrictive temperature. A control wild-type RK2 complex showed no inhibition of DNA synthesis at the restrictive temperature. (ii) Analysis of plasmid-encoded proteins revealed that the trfA-specified replication protein and other proteins which may be involved in the replication and maintenance of RK2 are located physically in the complex. Semiconservative plasmid DNA replication by the DNA-membrane complex was indicated by density shift experiments; DNA synthesized in the presence of a heavy-density precursor banded primarily in a heavier-density area of a neutral CsCl density gradient and consisted mostly of heavy- and light-density single-stranded DNA as determined by alkaline CsCl density gradient centrifugation. Plasmid RK2 DNA replication by the DNA-membrane complex appears to be coupled to transcription and translation as indicated by the following results: the inhibitory effects of chloramphenicol on both DNA and protein synthesis by the complex; the stimulation of replication by components normally required for protein synthesis (tRNA and all the common amino acids); the synthesis of RNA and protein by the complex; and the synthesis of specific RK2-encoded proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechhofer D. H., Figurski D. H. Map location and nucleotide sequence of korA, a key regulatory gene of promiscuous plasmid RK2. Nucleic Acids Res. 1983 Nov 11;11(21):7453–7469. doi: 10.1093/nar/11.21.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechhofer D. H., Kornacki J. A., Firshein W., Figurski D. H. Gene control in broad host range plasmid RK2: expression, polypeptide product, and multiple regulatory functions of korB. Proc Natl Acad Sci U S A. 1986 Jan;83(2):394–398. doi: 10.1073/pnas.83.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin P., Firshein W. Initiation of DNA replication in vitro by a DNA-membrane complex extracted from Bacillus subtilis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6214–6218. doi: 10.1073/pnas.80.20.6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin P., Strumph P., Kenny M., Firshein W. DNA synthesis in purified DNA-membrane complexes extracted from a Bacillus subtilis pol A mutant. Nature. 1982 Aug 19;298(5876):769–771. doi: 10.1038/298769a0. [DOI] [PubMed] [Google Scholar]
- Burkardt H. J., Riess G., Pühler A. Relationship of group P1 plasmids revealed by heteroduplex experiments: RP1, RP4, R68 and RK2 are identical. J Gen Microbiol. 1979 Oct;114(2):341–348. doi: 10.1099/00221287-114-2-341. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Pohlman R. F., Bechhofer D. H., Prince A. S., Kelton C. A. Broad host range plasmid RK2 encodes multiple kil genes potentially lethal to Escherichia coli host cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1935–1939. doi: 10.1073/pnas.79.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Young C., Schreiner H. C., Pohlman R. F., Bechhofer D. H., Prince A. S., D'Amico T. F. Genetic interactions of broad host-range plasmid RK2: evidence for a complex replication regulon. Basic Life Sci. 1985;30:227–241. doi: 10.1007/978-1-4613-2447-8_19. [DOI] [PubMed] [Google Scholar]
- Firshein W., Caro L. Detection of displacement ("D") loops with the properties of a replicating intermediate synthesized by a DNA/membrane complex derived from the low-copy-number plasmid RK2. Plasmid. 1984 Nov;12(3):227–232. doi: 10.1016/0147-619x(84)90051-9. [DOI] [PubMed] [Google Scholar]
- Firshein W., Strumph P., Benjamin P., Burnstein K., Kornacki J. Replication of a low-copy-number plasmid by a plasmid DNA-membrane complex extracted from minicells of Escherichia coli. J Bacteriol. 1982 Jun;150(3):1234–1243. doi: 10.1128/jb.150.3.1234-1243.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firshein W. The DNA-membrane fraction of Pneumococcus contains a DNA replication complex. J Mol Biol. 1972 Oct 14;70(3):383–397. doi: 10.1016/0022-2836(72)90547-5. [DOI] [PubMed] [Google Scholar]
- Inuzuka M., Helinski D. R. Replication of antibiotic resistance plasmid R6K DNA in vitro. Biochemistry. 1978 Jun 27;17(13):2567–2573. doi: 10.1021/bi00606a017. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Kornacki J. A., West A. H., Firshein W. Proteins encoded by the trans-acting replication and maintenance regions of broad host range plasmid RK2. Plasmid. 1984 Jan;11(1):48–57. doi: 10.1016/0147-619x(84)90006-4. [DOI] [PubMed] [Google Scholar]
- Leibowitz P. J., Schaechter M. The attachment of the bacterial chromosome to the cell membrane. Int Rev Cytol. 1975;41:1–28. doi: 10.1016/s0074-7696(08)60964-x. [DOI] [PubMed] [Google Scholar]
- Meyer R. J., Helinski D. R. Unidirectional replication of the P-group plasmid RK2. Biochim Biophys Acta. 1977 Sep 6;478(1):109–113. doi: 10.1016/0005-2787(77)90249-0. [DOI] [PubMed] [Google Scholar]
- Meyer R., Hinds M. Multiple mechanisms for expression of incompatibility by broad-host-range plasmid RK2. J Bacteriol. 1982 Dec;152(3):1078–1090. doi: 10.1128/jb.152.3.1078-1090.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Robinson M. K., Bennett P. M., Falkow S., Dodd H. M. Isolation of a temperature-sensitive derivative of RP1. Plasmid. 1980 May;3(3):343–347. doi: 10.1016/0147-619x(80)90047-5. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Rupert C. S. Determination of plasmid molecular weights from ultraviolet sensitivities. Nature. 1978 Mar 30;272(5652):471–472. doi: 10.1038/272471a0. [DOI] [PubMed] [Google Scholar]
- Schreiner H. C., Bechhofer D. H., Pohlman R. F., Young C., Borden P. A., Figurski D. H. Replication control in promiscuous plasmid RK2: kil and kor functions affect expression of the essential replication gene trfA. J Bacteriol. 1985 Jul;163(1):228–237. doi: 10.1128/jb.163.1.228-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingler V., Thomas C. M. Analysis of the trfA region of broad host-range plasmid RK2 by transposon mutagenesis and identification of polypeptide products. J Mol Biol. 1984 May 25;175(3):229–249. doi: 10.1016/0022-2836(84)90346-2. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Thomas C. M. Molecular gentic analysis of the trfB and korB region of broad host range plasmid RK2. J Gen Microbiol. 1984 Jul;130(7):1651–1663. doi: 10.1099/00221287-130-7-1651. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Thomas C. M. Nucleotide sequence of the trfA gene of broad host-range plasmid RK2. J Mol Biol. 1984 May 25;175(3):251–262. doi: 10.1016/0022-2836(84)90347-4. [DOI] [PubMed] [Google Scholar]
- Stalker D. M., Thomas C. M., Helinski D. R. Nucleotide sequence of the region of the origin of replication of the broad host range plasmid RK2. Mol Gen Genet. 1981;181(1):8–12. doi: 10.1007/BF00338997. [DOI] [PubMed] [Google Scholar]
- Thomas C. M. Complementation analysis of replication and maintenance functions of broad host range plasmids RK2 and RP1. Plasmid. 1981 May;5(3):277–291. doi: 10.1016/0147-619x(81)90005-6. [DOI] [PubMed] [Google Scholar]
- Thomas C. M., Meyer R., Helinski D. R. Regions of broad-host-range plasmid RK2 which are essential for replication and maintenance. J Bacteriol. 1980 Jan;141(1):213–222. doi: 10.1128/jb.141.1.213-222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. M. Molecular genetics of broad host range plasmid RK2. Plasmid. 1981 Jan;5(1):10–19. doi: 10.1016/0147-619x(81)90074-3. [DOI] [PubMed] [Google Scholar]
- Thomas C. M., Stalker D. M., Helinski D. R. Replication and incompatibility properties of segments of the origin region of replication of the broad host range plasmid RK2. Mol Gen Genet. 1981;181(1):1–7. doi: 10.1007/BF00338996. [DOI] [PubMed] [Google Scholar]
- Tremblay G. Y., Daniels M. J., Schaechter M. Isolation of a cell membrane-DNA-nascent RNA complex from bacteria. J Mol Biol. 1969 Feb 28;40(1):65–76. doi: 10.1016/0022-2836(69)90296-4. [DOI] [PubMed] [Google Scholar]
- Witholt B., Heerikhuizen H. V., De Leij L. How does lysozyme penetrate through the bacterial outer membrane? Biochim Biophys Acta. 1976 Sep 7;443(3):534–544. doi: 10.1016/0005-2736(76)90471-5. [DOI] [PubMed] [Google Scholar]