Abstract
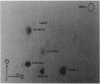
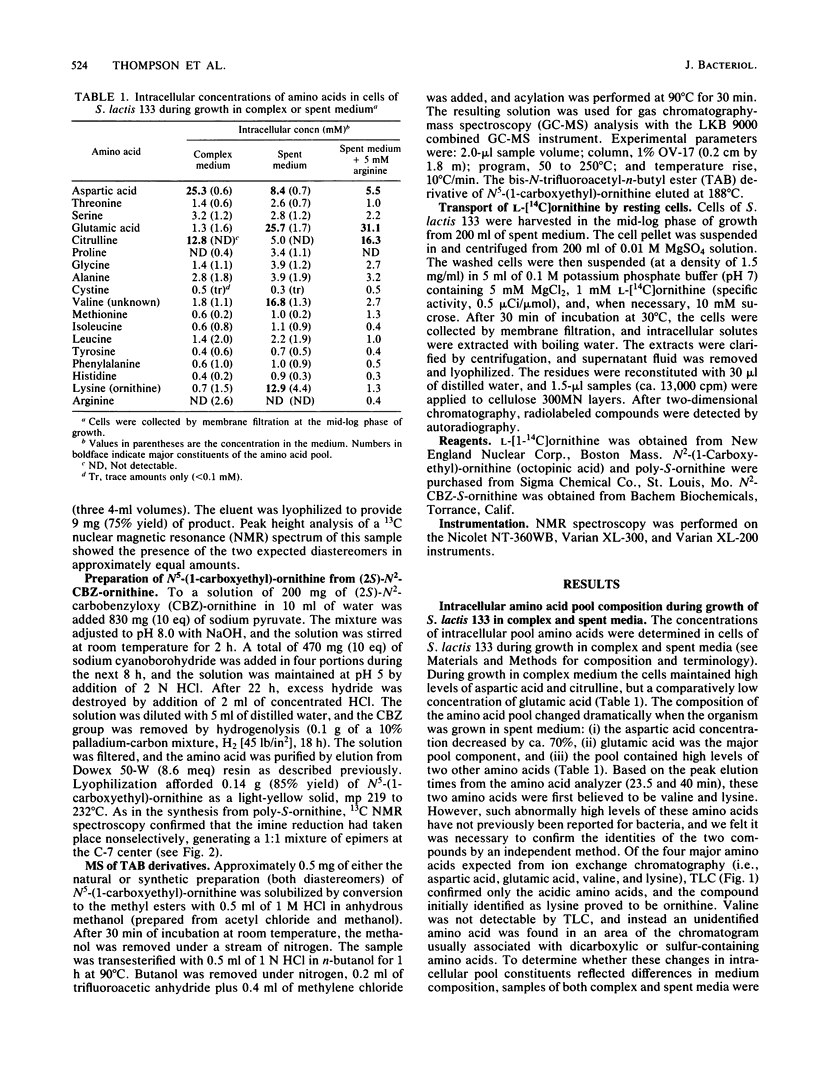
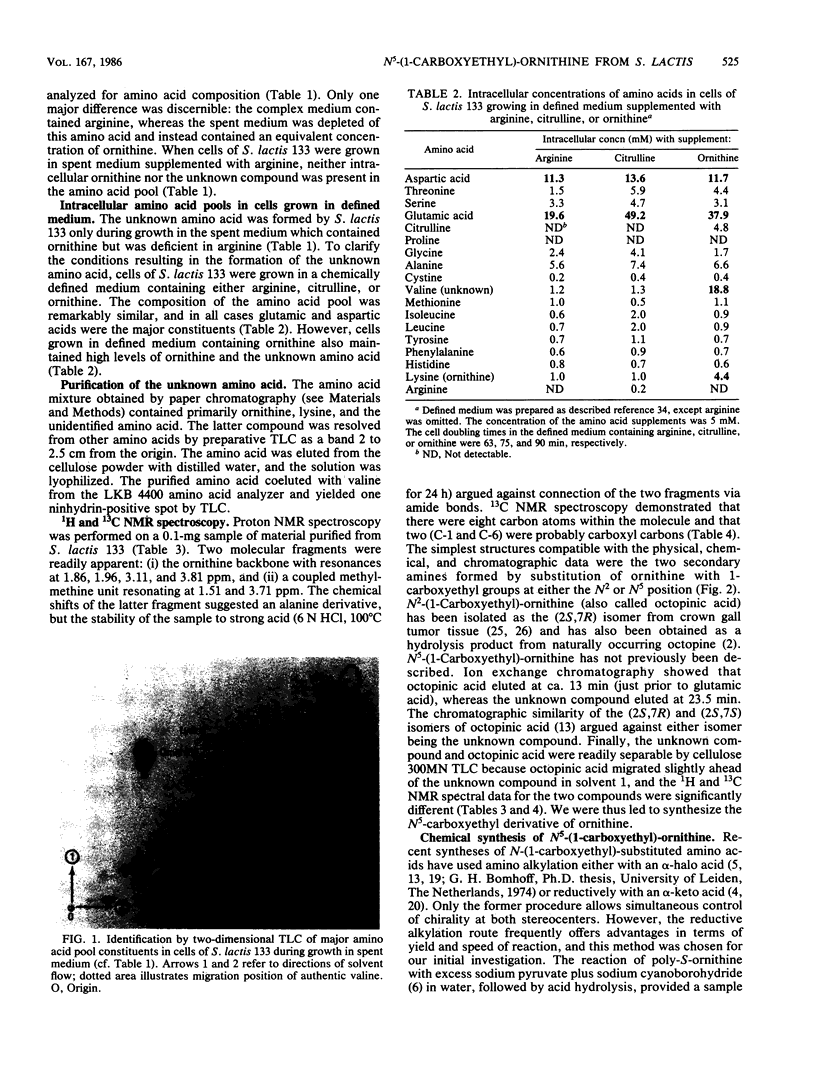
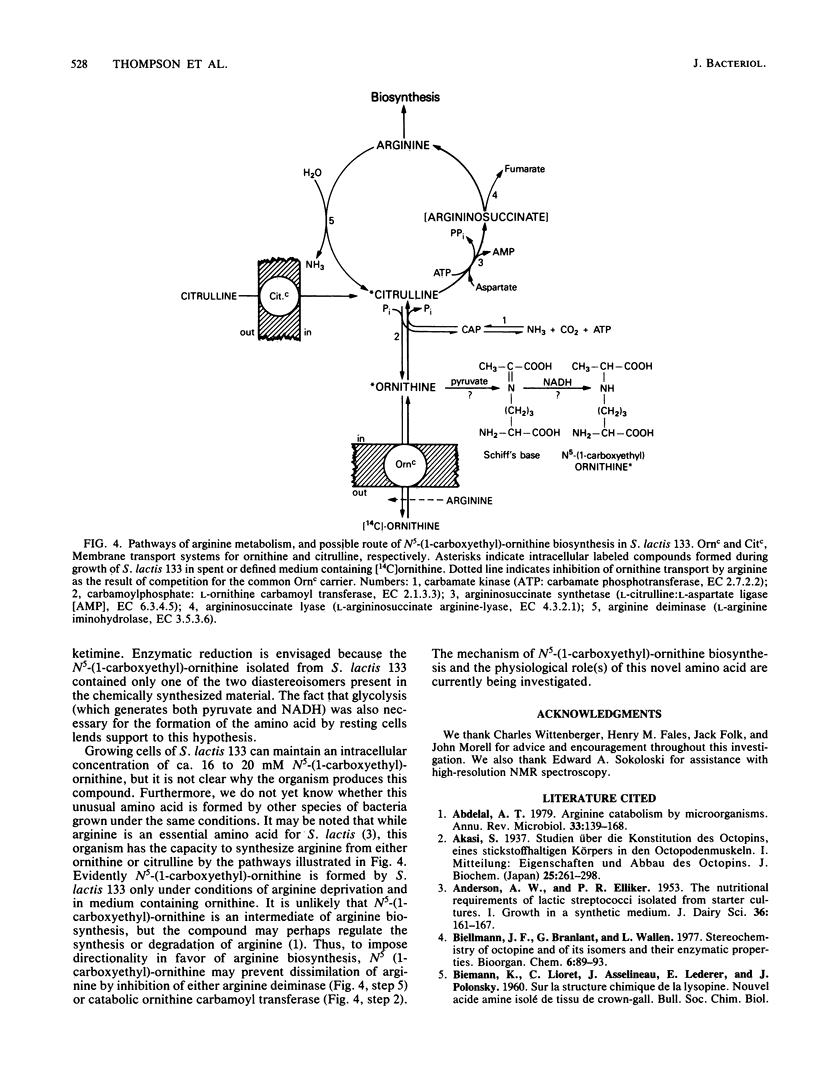
Intracellular concentrations of amino acids were determined in cells of Streptococcus lactis 133 during growth in complex, spent, and chemically defined media. Glutamic and aspartic acids represented the major constituents of the amino acid pool. However, organisms grown in spent medium or in defined medium supplemented with ornithine also contained unusually high levels of two additional amino acids. One of these amino acids was ornithine. The second compound exhibited properties of a neutral amino acid by coelution with valine from the amino acid analyzer. The compound did not, however, comigrate with valine or any other standard amino acid by two-dimensional thin-layer chromatography. The unknown amino acid was purified by paper and thin-layer chromatography, and its molecular structure was determined by 1H and 13C nuclear magnetic resonance spectroscopy. This new amino acid was shown to be N5-(1-carboxyethyl)-ornithine. The 14C-labeled compound was formed by cells of S. lactis 133 during growth in spent medium or defined medium containing [14C]ornithine. Formation of the derivative by resting cells required ornithine and the presence of a metabolizable sugar. N5-(1-Carboxyethyl)-ornithine was synthesized chemically from both poly-S-ornithine and (2S)-N2-carbobenzyloxy-ornithine as a 1:1 mixture of two diastereomers. The physical and chemical properties of the amino acid purified from S. lactis 133 were identical to those of one of the synthetic diastereomers. The bis-N-trifluoroacetyl-di-n-butyl esters of the natural and synthetic compounds generated identical gas chromatography-mass spectrometry spectra. A mechanism is suggested for the in vivo synthesis of N5-(1-carboxyethyl)-ornithine, and the possible functions of this new amino acid are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T. Arginine catabolism by microorganisms. Annu Rev Microbiol. 1979;33:139–168. doi: 10.1146/annurev.mi.33.100179.001035. [DOI] [PubMed] [Google Scholar]
- Clark V. L., Peterson D. E., Bernlohr R. W. Changes in free amino acid production and intracellular amino acid pools of Bacillus licheniformis as a function of culture age and growth media. J Bacteriol. 1972 Nov;112(2):715–725. doi: 10.1128/jb.112.2.715-725.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith C. J., Melville T. H. Influence of growth conditions on the composition of some streptococcal amino acid pools. Microbios. 1974 Jan;9(33):7–13. [PubMed] [Google Scholar]
- HOLDEN J. T., HOLMAN J. Accumulation of freely extractable glutamic acid by lactic acid bacteria. J Biol Chem. 1959 Apr;234(4):865–871. [PubMed] [Google Scholar]
- Hack E., Kemp J. D. Purification and Characterization of the Crown Gall-specific Enzyme, Octopine Synthase. Plant Physiol. 1980 May;65(5):949–955. doi: 10.1104/pp.65.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan N. J., Midgley M., Dawes E. A. Effect of starvation on transport, membrane potential and survival of Staphylococcus epidermidis under anaerobic conditions. J Gen Microbiol. 1981 Dec;127(2):223–230. doi: 10.1099/00221287-127-2-223. [DOI] [PubMed] [Google Scholar]
- Jensen R. E., Zdybak W. T., Yasuda K., Chilton W. S. A useful synthesis of nopaline, a crown gall tumor metabolite. Biochem Biophys Res Commun. 1977 Apr 25;75(4):1066–1070. doi: 10.1016/0006-291x(77)91490-5. [DOI] [PubMed] [Google Scholar]
- Kemp J. D. A new amino acid derivative present in crown gall tumor tissue. Biochem Biophys Res Commun. 1977 Feb 7;74(3):862–868. doi: 10.1016/0006-291x(77)91598-4. [DOI] [PubMed] [Google Scholar]
- MENAGE A., MOREL G. SUR LA PR'ESENCE D'OCTOPINE DANS LES TISSUS DE CROWN-GALL. C R Hebd Seances Acad Sci. 1964 Dec 21;259:4795–4796. [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. 18. Free amino acids in spores. J Biol Chem. 1970 Mar 10;245(5):1128–1136. [PubMed] [Google Scholar]
- Pillay D. T., Mehdi R. Separation of amino acids by thin-layer chromatography. J Chromatogr. 1970 Feb 18;47(1):119–123. doi: 10.1016/0021-9673(70)80018-8. [DOI] [PubMed] [Google Scholar]
- Stalling D. L., Gehrke C. W. Quantitative analysis of amino acids by gas chromatography: acylation of arginine. Biochem Biophys Res Commun. 1966 Feb 3;22(3):329–335. doi: 10.1016/0006-291x(66)90486-4. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970 Dec;64(2):171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Degradation of cell constituents by starved Streptococcus lactis in relation to survival. J Gen Microbiol. 1969 Nov;58(3):347–362. doi: 10.1099/00221287-58-3-347. [DOI] [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. In vivo regulation of glycolysis and characterization of sugar: phosphotransferase systems in Streptococcus lactis. J Bacteriol. 1978 Nov;136(2):465–476. doi: 10.1128/jb.136.2.465-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Saier M. H., Jr Regulation of methyl-beta-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981 Jun;146(3):885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Torchia D. A. Use of 31P nuclear magnetic resonance spectroscopy and 14C fluorography in studies of glycolysis and regulation of pyruvate kinase in Streptococcus lactis. J Bacteriol. 1984 Jun;158(3):791–800. doi: 10.1128/jb.158.3.791-800.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]