Abstract
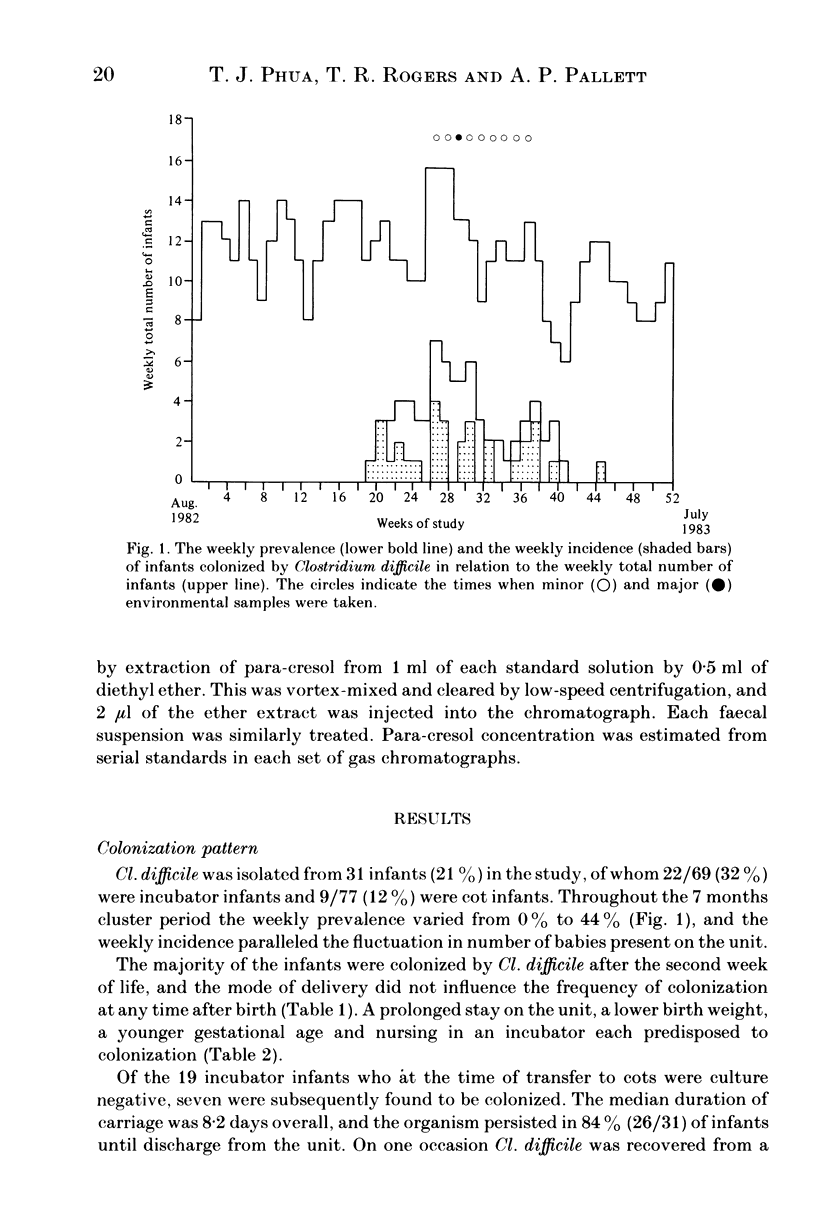
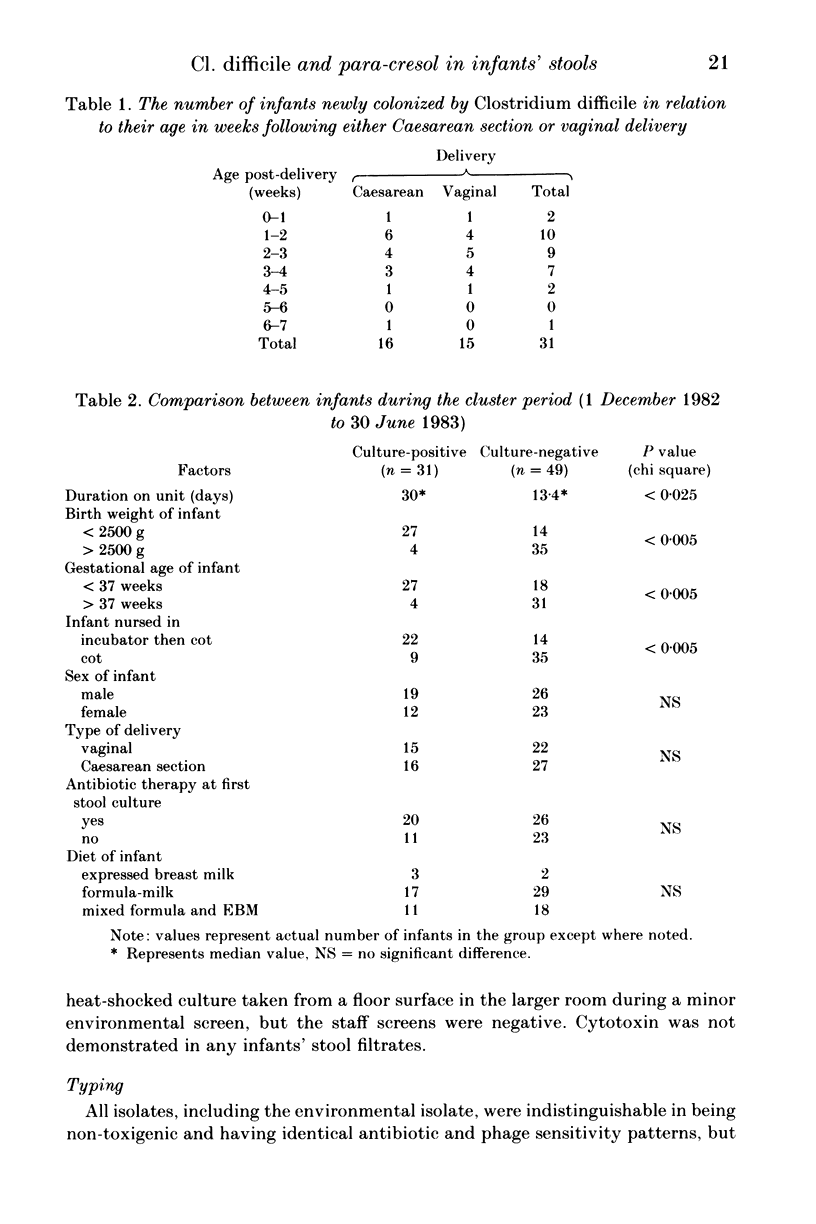
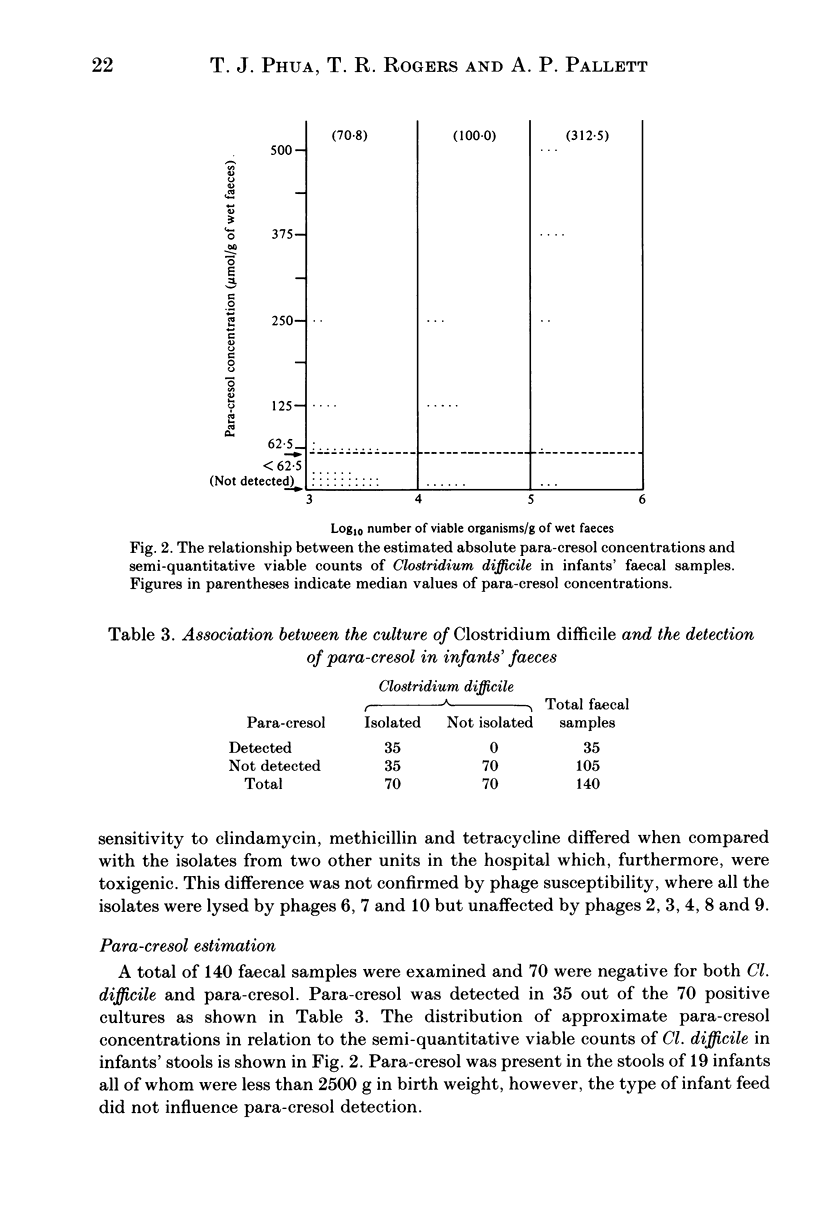
Infants' stools were examined for the presence of Clostridium difficile and its cytotoxin in a study performed over a one-year period on a special care baby unit. Overall, 21% of infants were colonized, but the organism was only recovered in a seven-month period during which its weekly prevalence in the group varied from zero to 44%, with a distinct clustering of colonized infants being observed. Tests for the presence of cytotoxin in the stools and in supernatants of broth that had been inoculated with each isolate were negative. The factors predisposing to colonization were a prolonged stay in the unit, low birth weight, younger gestational age and being nursed in an incubator. The organism was recovered only once from an environmental screen. An antibiogram, used in conjunction with toxin production, was helpful in distinguishing these isolates from a collection obtained from other units in the hospital. We conclude that Cl. difficile was acquired by nosocomial spread although we did not establish the precise mechanism involved. The detection of para-cresol by gas-liquid chromatography was found to be specific but insufficiently sensitive as a screening test for the organism's presence in the stools. It could only be demonstrated in infants whose birth-weights were less than 2500 g, and no association was observed between the type of feed and para-cresol presence in stools.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bone E., Tamm A., Hill M. The production of urinary phenols by gut bacteria and their possible role in the causation of large bowel cancer. Am J Clin Nutr. 1976 Dec;29(12):1448–1454. doi: 10.1093/ajcn/29.12.1448. [DOI] [PubMed] [Google Scholar]
- Cashore W. J., Peter G., Lauermann M., Stonestreet B. S., Oh W. Clostridia colonization and clostridial toxin in neonatal necrotizing enterocolitis. J Pediatr. 1981 Feb;98(2):308–311. doi: 10.1016/s0022-3476(81)80667-1. [DOI] [PubMed] [Google Scholar]
- Cooperstock M., Riegle L., Woodruff C. W., Onderdonk A. Influence of age, sex, and diet on asymptomatic colonization of infants with Clostridium difficile. J Clin Microbiol. 1983 May;17(5):830–833. doi: 10.1128/jcm.17.5.830-833.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsden S. R., Hilton M. G., Waller J. M. The end products of the metabolism of aromatic amino acids by Clostridia. Arch Microbiol. 1976 Apr 1;107(3):283–288. doi: 10.1007/BF00425340. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Menda K. B., Thadepalli H., Keith L. Anaerobic microflora of the cervix in healthy women. Am J Obstet Gynecol. 1973 Dec 15;117(8):1053–1055. doi: 10.1016/0002-9378(73)90753-9. [DOI] [PubMed] [Google Scholar]
- Greenfield C., Aguilar Ramirez J. R., Pounder R. E., Williams T., Danvers M., Marper S. R., Noone P. Clostridium difficile and inflammatory bowel disease. Gut. 1983 Aug;24(8):713–717. doi: 10.1136/gut.24.8.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafiz S., Oakley C. L. Clostridium difficile: isolation and characteristics. J Med Microbiol. 1976 May;9(2):129–136. doi: 10.1099/00222615-9-2-129. [DOI] [PubMed] [Google Scholar]
- Larson H. E., Barclay F. E., Honour P., Hill I. D. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982 Dec;146(6):727–733. doi: 10.1093/infdis/146.6.727. [DOI] [PubMed] [Google Scholar]
- Larson H. E., Price A. B., Honour P., Borriello S. P. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978 May 20;1(8073):1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- Malamou-Ladas H., O'Farrell S., Nash J. Q., Tabaqchali S. Isolation of Clostridium difficile from patients and the environment of hospital wards. J Clin Pathol. 1983 Jan;36(1):88–92. doi: 10.1136/jcp.36.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips K. D., Rogers P. A. Rapid detection and presumptive identification of Clostridium difficile by p-cresol production on a selective medium. J Clin Pathol. 1981 Jun;34(6):642–644. doi: 10.1136/jcp.34.6.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvliege C., Labbé M., Yourassowsky E. Gas-liquid chromatography as screening test for Clostridium difficile. Lancet. 1981 Nov 14;2(8255):1105–1105. doi: 10.1016/s0140-6736(81)91296-4. [DOI] [PubMed] [Google Scholar]
- Rogers T. R., Petrou M., Lucas C., Chung J. T., Barrett A. J., Borriello S. P., Honour P. Spread of Clostridium difficile among patients receiving non-absorbable antibiotics for gut decontamination. Br Med J (Clin Res Ed) 1981 Aug 8;283(6288):408–409. doi: 10.1136/bmj.283.6288.408-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell T. L., Schaberg D. R., Fekety F. R. Bacteriophage and bacteriocin typing scheme for Clostridium difficile. J Clin Microbiol. 1983 Jun;17(6):1148–1152. doi: 10.1128/jcm.17.6.1148-1152.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherertz R. J., Sarubbi F. A. The prevalence of Clostridium difficile and toxin in a nursery population: a comparison between patients with necrotizing enterocolitis and an asymptomatic group. J Pediatr. 1982 Mar;100(3):435–439. doi: 10.1016/s0022-3476(82)80455-1. [DOI] [PubMed] [Google Scholar]
- Stark P. L., Lee A. Clostridia isolated from the feces of infants during the first year of life. J Pediatr. 1982 Mar;100(3):362–365. doi: 10.1016/s0022-3476(82)80430-7. [DOI] [PubMed] [Google Scholar]
- Viscidi R., Willey S., Bartlett J. G. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology. 1981 Jul;81(1):5–9. [PubMed] [Google Scholar]
- Wysowski D. K., Flynt J. W., Jr, Goldfield M., Altman R., Davis A. T. Epidemic neonatal hyperbilirubinemia and use of a phenolic disinfectant detergent. Pediatrics. 1978 Feb;61(2):165–170. [PubMed] [Google Scholar]
- Yokoyama M. T., Tabori C., Miller E. R., Hogberg M. G. The effects of antibiotics in the weanling pig diet on growth and the excretion of volatile phenolic and aromatic bacterial metabolites. Am J Clin Nutr. 1982 Jun;35(6):1417–1424. doi: 10.1093/ajcn/35.6.1417. [DOI] [PubMed] [Google Scholar]


