Abstract
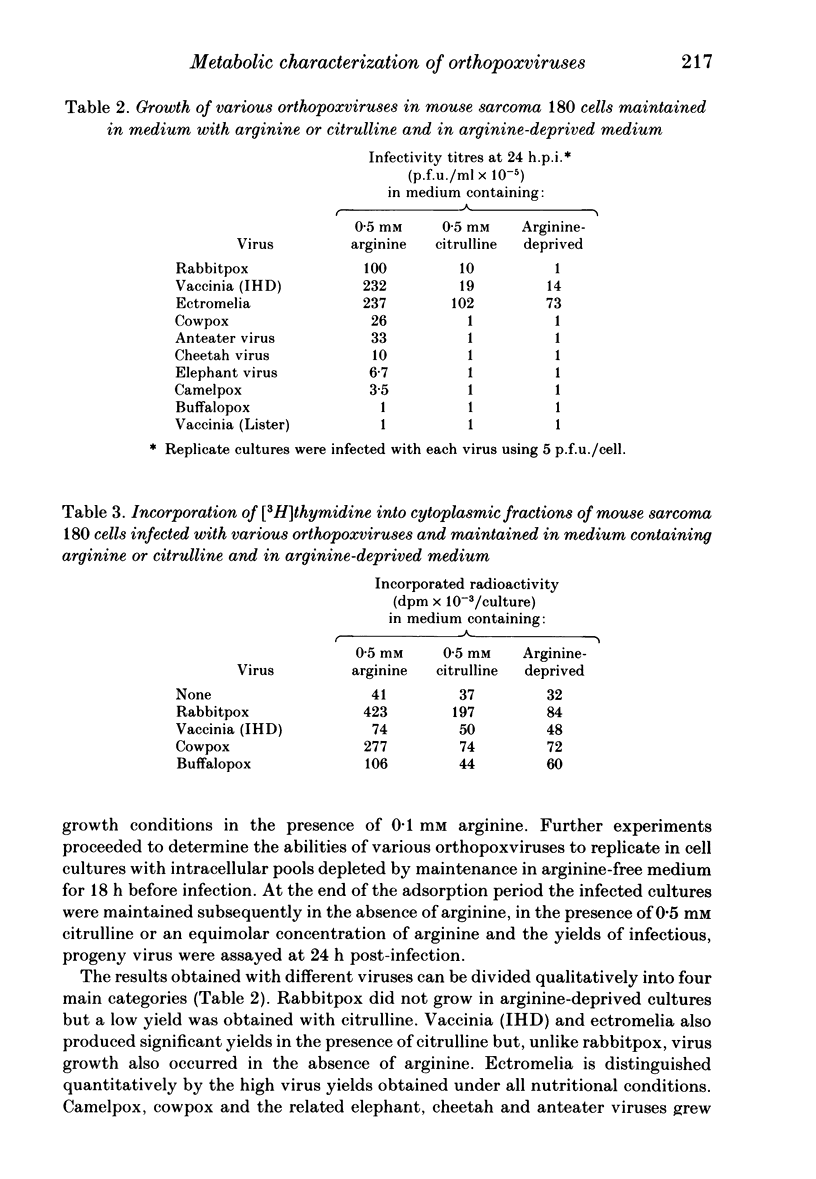
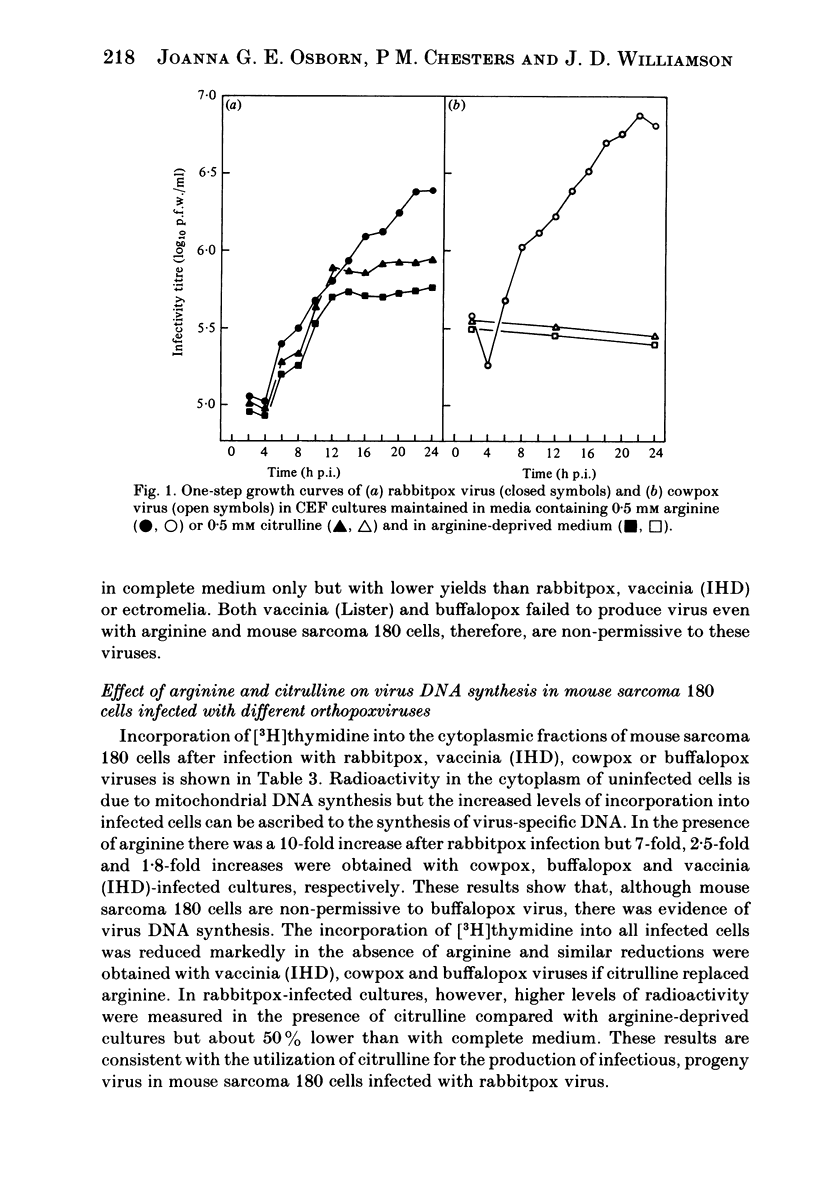
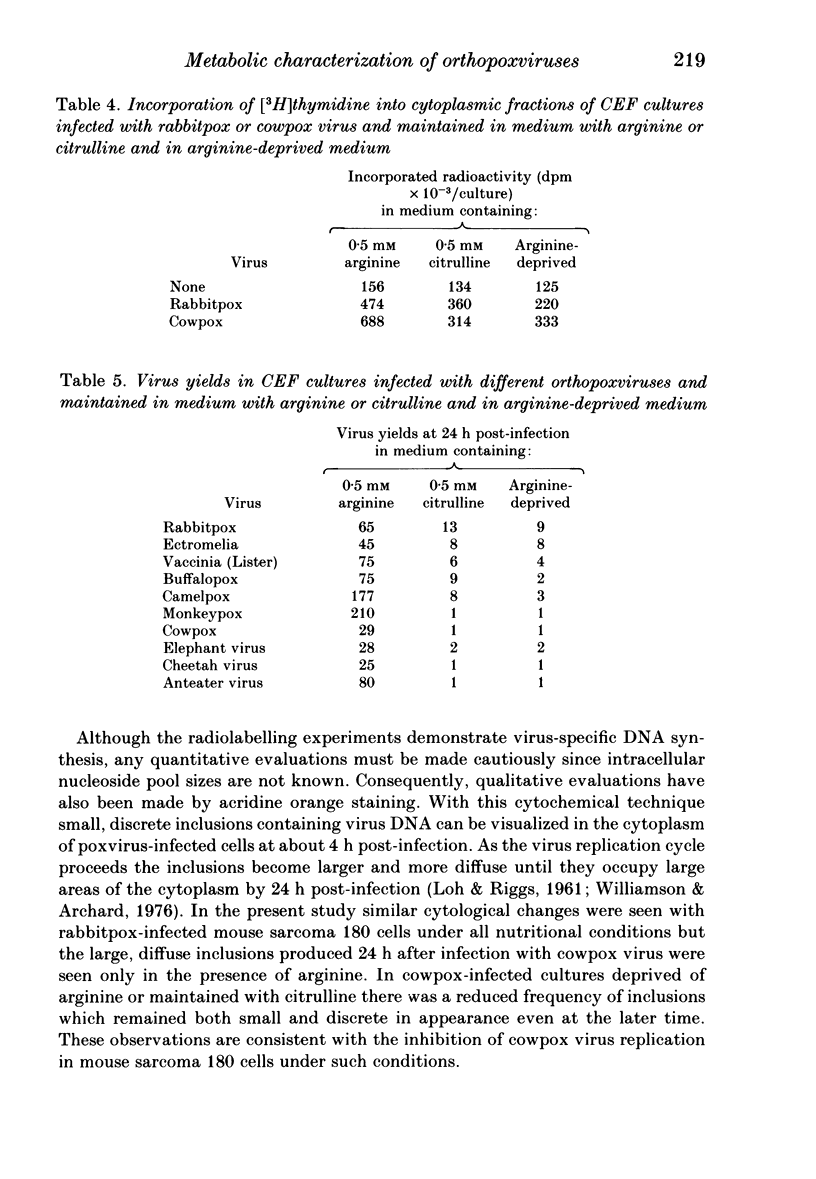
Arginine has been shown to be essential for the replication of several orthopoxviruses in mouse sarcoma 180 cells and in chick embryo fibroblast cultures. Both host systems are characterized by their inabilities to utilize citrulline for the biosynthesis of arginine due to deficiencies in the requisite cellular enzymes and cell multiplication is absolutely dependent on the availability of exogenous arginine. Virus replication in such cells maintained with citrulline results from the induction of virus-specific enzymes. Significant virus yields in the absence of exogenous arginine or citrulline can arise from the replenishment of intracellular amino acid pools by increased utilization of arginyl residues in cellular proteins. The extent of the phenotypic expression of these characters in infected cells permitted significant discrimination between the viruses examined. Distinctions could be drawn between rabbitpox, ectromelia, cowpox, buffalopox and vaccinia strains. However, cowpox could not be distinguished from other viruses isolated from diseased animals in European zoos.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archard L. C., Williamson J. D. The effect of arginine deprivation on the replication of vaccinia virus. J Gen Virol. 1971 Sep;12(3):249–258. doi: 10.1099/0022-1317-12-3-249. [DOI] [PubMed] [Google Scholar]
- Baxby D., Ashton D. G., Jones D., Thomsett L. R., Denham E. M. Cowpox virus infection in unusual hosts. Vet Rec. 1979 Feb 24;104(8):175–175. doi: 10.1136/vr.104.8.175-a. [DOI] [PubMed] [Google Scholar]
- Baxby D., Ghaboosi B. Laboratory characteristics of poxviruses isolated from captive elephants in Germany. J Gen Virol. 1977 Nov;37(2):407–414. doi: 10.1099/0022-1317-37-2-407. [DOI] [PubMed] [Google Scholar]
- Baxby D. Identification and interrelationships of the variola/vaccinia subgroup of poxviruses. Prog Med Virol. 1975;19:215–246. [PubMed] [Google Scholar]
- Baxby D. Is cowpox misnamed? A review of 10 human cases. Br Med J. 1977 May 28;1(6073):1379–1381. doi: 10.1136/bmj.1.6073.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxby D., Shackleton W. B., Wheeler J., Turner A. Comparison of cowpox-like viruses isolated from European zoos. Brief report. Arch Virol. 1979;61(4):337–340. doi: 10.1007/BF01315021. [DOI] [PubMed] [Google Scholar]
- Bedson H. S. Enzyme studies for the characterization of some orthopoxvirus isolates. Bull World Health Organ. 1982;60(3):377–380. [PMC free article] [PubMed] [Google Scholar]
- Breman J. G., Kalisa-Ruti, Steniowski M. V., Zanotto E., Gromyko A. I., Arita I. Human monkeypox, 1970-79. Bull World Health Organ. 1980;58(2):165–182. [PMC free article] [PubMed] [Google Scholar]
- Cooke B. C., Williamson J. D. Enhanced utilization of citrulline in rabbitpox virus-infected mouse sarcoma 180 cells. J Gen Virol. 1973 Nov;21(2):339–348. doi: 10.1099/0022-1317-21-2-339. [DOI] [PubMed] [Google Scholar]
- Harper L., Bedson H. S., Buchan A. Identification of orthopoxviruses by polyacrylamide gel electrophoresis of intracellular polypeptides. I. Four major groupings. Virology. 1979 Mar;93(2):435–444. doi: 10.1016/0042-6822(79)90247-2. [DOI] [PubMed] [Google Scholar]
- Kataria R. S., Singh I. P. Serological relationship of buffalopox virus to vaccinia and cowpox viruses. Acta Virol. 1970 Jul;14(4):307–311. [PubMed] [Google Scholar]
- LOH P. C., RIGGS J. L. Demonstration of the sequential development of vaccinial antigens and virus in infected cells: observations with cytochemical and differential fluorescent procedures. J Exp Med. 1961 Jul 1;114:149–160. doi: 10.1084/jem.114.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Archard L. C. Conservation and variation in Orthopoxvirus genome structure. J Gen Virol. 1979 Dec;45(3):683–701. doi: 10.1099/0022-1317-45-3-683. [DOI] [PubMed] [Google Scholar]
- Marennikova S. S., Maltseva N. N., Korneeva V. I., Garanina N. Outbreak of pox disease among carnivora (felidae) and edentata. J Infect Dis. 1977 Mar;135(3):358–366. doi: 10.1093/infdis/135.3.358. [DOI] [PubMed] [Google Scholar]
- Obert G., Tripier F., Bingen A., Guir J. Effets de la carence en arginine sur la réplication du virus vaccinal. C R Acad Sci Hebd Seances Acad Sci D. 1971 Mar 22;272(12):1705–1708. [PubMed] [Google Scholar]
- TYTELL A. A., NEUMAN R. E. Growth response of stable and primary cell cultures to L-ornithine, L-citrulline, and L-arginine. Exp Cell Res. 1960 Jun;20:84–91. doi: 10.1016/0014-4827(60)90225-1. [DOI] [PubMed] [Google Scholar]
- Turner A., Baxby D. Structural polypeptides of Orthopoxvirus: their distribution in various members and location within the virion. J Gen Virol. 1979 Dec;45(3):537–545. doi: 10.1099/0022-1317-45-3-537. [DOI] [PubMed] [Google Scholar]
- Williamson J. D., Archard L. C. The effect of canaline on some events in vaccinia virus replication. J Gen Virol. 1976 Jan;30(1):81–89. doi: 10.1099/0022-1317-30-1-81. [DOI] [PubMed] [Google Scholar]
- Williamson J. D., Cooke B. C. Argininosuccinate synthetase-lyase activity in vaccinia virus-infected HeLa and mouse L cells. J Gen Virol. 1973 Nov;21(2):349–357. doi: 10.1099/0022-1317-21-2-349. [DOI] [PubMed] [Google Scholar]
- Williamson J. D., Mackett M. Arginine deprivation and the generation of white variants in cowpox virus-infected cell cultures. J Hyg (Lond) 1982 Dec;89(3):373–381. doi: 10.1017/s0022172400070947. [DOI] [PMC free article] [PubMed] [Google Scholar]


