Abstract
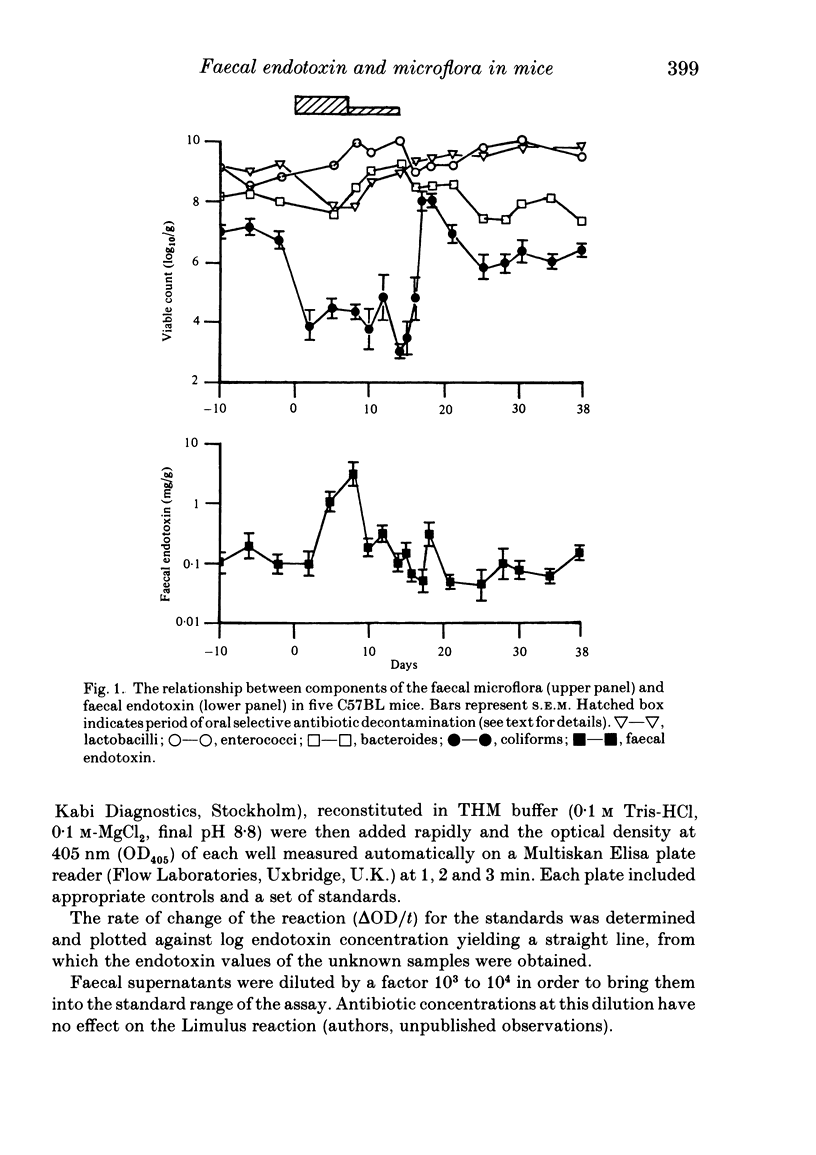
We studied the effect of oral selective antibiotic decontamination (SD) on the faecal endotoxin content and microflora in individual C57BL mice. Suppression of the coliform count was associated with an initial rise in faecal endotoxin concentration from 0.1 to 3.1 mg/g wet faeces during the first week of SD, which fell to 0.04 mg/g during the second week of treatment. Cessation of SD resulted in an immediate sharp increase in coliform count followed by its decline and gradual recovery to pre-treatment counts. Faecal endotoxin levels followed a parallel course. SD did not effect significantly the counts of lactobacilli, bacteroides and enterococci. It appears that the coliform population is responsible for the overall level of faecal endotoxin, and that during the initial period of SD endotoxin levels are elevated, an effect which may be mediated by antibiotic-enhanced release of endotoxin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. M., Solberg O. The endotoxin-liberating effect of antibiotics on meningococci in vitro. Acta Pathol Microbiol Scand B. 1980 Aug;88(4):231–236. doi: 10.1111/j.1699-0463.1980.tb02633.x. [DOI] [PubMed] [Google Scholar]
- Cohen J., McConnell J. S. Observations on the measurement and evaluation of endotoxemia by a quantitative limulus lysate microassay. J Infect Dis. 1984 Dec;150(6):916–924. doi: 10.1093/infdis/150.6.916. [DOI] [PubMed] [Google Scholar]
- Crowther J. S. Transport and storage of faeces for bacteriological examination. J Appl Bacteriol. 1971 Jun;34(2):477–483. doi: 10.1111/j.1365-2672.1971.tb02307.x. [DOI] [PubMed] [Google Scholar]
- Keast D. Role of bacterial endotoxin in the graft-vs.-host syndrome. J Infect Dis. 1973 Jul;128(Suppl):104–109. doi: 10.1093/infdis/128.supplement_1.s104. [DOI] [PubMed] [Google Scholar]
- Lee A., Gordon J., Lee C. J., Dubos R. The mouse intestinal microflora with emphasis on the strict anaerobes. J Exp Med. 1971 Feb 1;133(2):339–352. doi: 10.1084/jem.133.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- RAVIN H. A., ROWLEY D., JENKINS C., FINE J. On the absorption of bacterial endotoxin from the gastro-intestinal tract of the normal and shocked animal. J Exp Med. 1960 Nov 1;112:783–792. doi: 10.1084/jem.112.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Schade U., Jensen M., Wollenweber H. W., Lüderitz O., Greisman S. G. Bacterial endotoxins: chemical structure, biological activity and role in septicaemia. Scand J Infect Dis Suppl. 1982;31:8–21. [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R. J. The fecal flora of various strains of mice. Its bearing on their susceptibility to endotoxin. J Exp Med. 1962 Jun 1;115:1149–1160. doi: 10.1084/jem.115.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep J. L., Mogan K. A. Kinetics of endotoxin release during antibiotic therapy for experimental gram-negative bacterial sepsis. J Infect Dis. 1984 Sep;150(3):380–388. doi: 10.1093/infdis/150.3.380. [DOI] [PubMed] [Google Scholar]
- Skopińska E. Some effects of Escherichia coli endotoxin on the graft-versus-host reaction in mice. Transplantation. 1972 Oct;14(4):432–437. [PubMed] [Google Scholar]
- Walker R. I., Ledney G. D., Galley C. B. Aseptic endotoxemia in radiation injury and graft-vs-host diesease. Radiat Res. 1975 May;62(2):242–249. [PubMed] [Google Scholar]
- van Bekkum D. W., Knaan S. Role of bacterial microflora in development of intestinal lesions from graft-versus-host reaction. J Natl Cancer Inst. 1977 Mar;58(3):787–790. doi: 10.1093/jnci/58.3.787. [DOI] [PubMed] [Google Scholar]
- van Bekkum D. W., Roodenburg J., Heidt P. J., van der Waaij D. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J Natl Cancer Inst. 1974 Feb;52(2):401–404. doi: 10.1093/jnci/52.2.401. [DOI] [PubMed] [Google Scholar]
- van der Waaij D., Sturm C. A. Antibiotic decontimination of the digestive tract of mice. Technical procedures. Lab Anim Care. 1968 Feb;18(1):1–10. [PubMed] [Google Scholar]
- van der Waaij D. The persistent absence of Enterobacteriaceae from the intestinal flora of mice following antibiotic treatment. J Infect Dis. 1968 Feb;118(1):32–38. doi: 10.1093/infdis/118.1.32. [DOI] [PubMed] [Google Scholar]


