Abstract
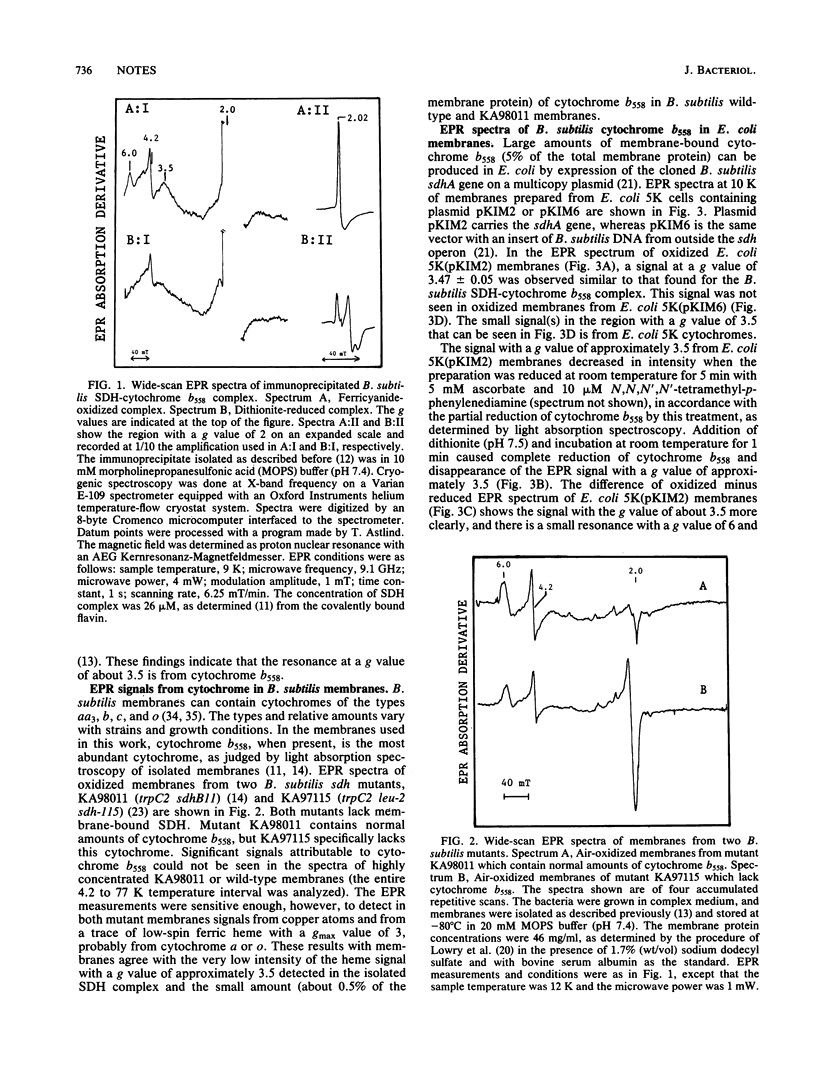
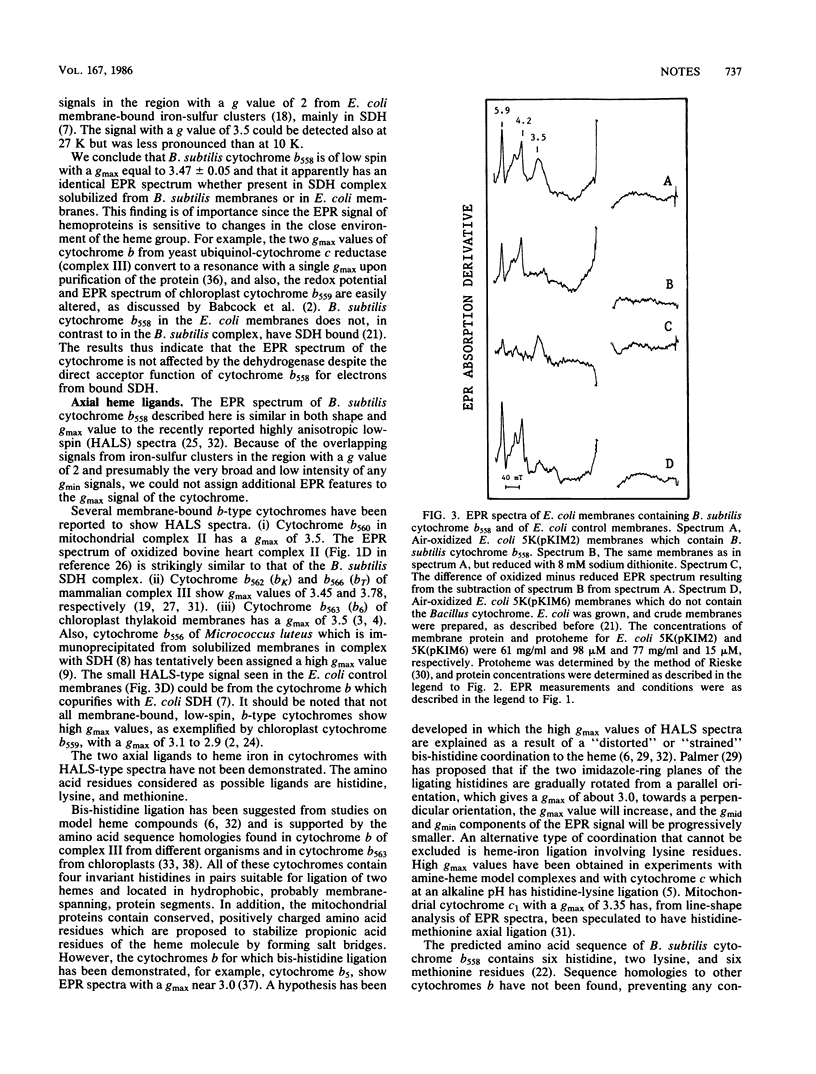
Cytochrome b558 of the Bacillus subtilis succinate dehydrogenase complex was studied by electron-paramagnetic-resonance (EPR) spectroscopy. The cytochrome amplified in Escherichia coli membranes by expression of the cloned cytochrome gene and in the succinate dehydrogenase complex immunoprecipitated from solubilized B. subtilis membranes, respectively, is shown to be low spin with a highly anisotropic (gmax approximately equal to 3.5) EPR signal. The amino acid residues most likely forming fifth and sixth axial ligands to heme in cytochrome b558 are discussed on the basis of the EPR signal and the recently determined gene sequence (K. Magnusson, M. Philips, J.R. Guest, and L. Rutberg, J. Bacteriol. 166:1067-1071, 1986) and in comparison with other b-type cytochromes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson K. K., Lipscomb J. D., Valentine M., Münck E., Hooper A. B. Tetraheme cytochrome c-554 from Nitrosomonas europaea. Heme-heme interactions and ligand binding. J Biol Chem. 1986 Jan 25;261(3):1126–1138. [PubMed] [Google Scholar]
- Babcock G. T., Widger W. R., Cramer W. A., Oertling W. A., Metz J. G. Axial ligands of chloroplast cytochrome b-559: identification and requirement for a heme-cross-linked polypeptide structure. Biochemistry. 1985 Jul 2;24(14):3638–3645. doi: 10.1021/bi00335a036. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Feinberg B. A., Hoffman B. M., Margoliash E., Preisach J., Blumberg W. E. Multiple low spin forms of the cytochrome c ferrihemochrome. EPR spectra of various eukaryotic and prokaryotic cytochromes c. J Biol Chem. 1977 Jan 25;252(2):574–582. [PubMed] [Google Scholar]
- Carter K. R., Tsai A., Palmer G. The coordination environment of mitochondrial cytochromes b. FEBS Lett. 1981 Sep 28;132(2):243–246. doi: 10.1016/0014-5793(81)81170-2. [DOI] [PubMed] [Google Scholar]
- Condon C., Cammack R., Patil D. S., Owen P. The succinate dehydrogenase of Escherichia coli. Immunochemical resolution and biophysical characterization of a 4-subunit enzyme complex. J Biol Chem. 1985 Aug 5;260(16):9427–9434. [PubMed] [Google Scholar]
- Crowe B. A., Owen P., Cammack R. Study of the respiratory chain in Micrococcus luteus (lysodeikticus) by electron-spin-resonance spectroscopy. Eur J Biochem. 1983 Dec 1;137(1-2):185–190. doi: 10.1111/j.1432-1033.1983.tb07813.x. [DOI] [PubMed] [Google Scholar]
- Crowe B. A., Owen P. Molecular properties of succinate dehydrogenase isolated from Micrococcus luteus (lysodeikticus). J Bacteriol. 1983 Mar;153(3):1493–1501. doi: 10.1128/jb.153.3.1493-1501.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Hederstedt L. Cytochrome b reducible by succinate in an isolated succinate dehydrogenase-cytochrome b complex from Bacillus subtilis membranes. J Bacteriol. 1980 Dec;144(3):933–940. doi: 10.1128/jb.144.3.933-940.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Holmgren E., Rutberg L. Characterization of a succinate dehydrogenase complex solubilized from the cytoplasmic membrane of Bacillus subtilis with the nonionic detergent Triton X-100. J Bacteriol. 1979 May;138(2):370–376. doi: 10.1128/jb.138.2.370-376.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Maguire J. J., Waring A. J., Ohnishi T. Characterization by electron paramagnetic resonance and studies on subunit location and assembly of the iron-sulfur clusters of Bacillus subtilis succinate dehydrogenase. J Biol Chem. 1985 May 10;260(9):5554–5562. [PubMed] [Google Scholar]
- Hederstedt L., Rutberg L. Biosynthesis and membrane binding of succinate dehydrogenase in Bacillus subtilis. J Bacteriol. 1980 Dec;144(3):941–951. doi: 10.1128/jb.144.3.941-951.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Rutberg L. Orientation of succinate dehydrogenase and cytochrome b558 in the Bacillus subtilis cytoplasmic membrane. J Bacteriol. 1983 Jan;153(1):57–65. doi: 10.1128/jb.153.1.57-65.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Rutberg L. Succinate dehydrogenase--a comparative review. Microbiol Rev. 1981 Dec;45(4):542–555. doi: 10.1128/mr.45.4.542-555.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren E., Hederstedt L., Rutberg L. Role of heme in synthesis and membrane binding of succinic dehydrogenase in Bacillus subtilis. J Bacteriol. 1979 May;138(2):377–382. doi: 10.1128/jb.138.2.377-382.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J., Reid G. A., Poole R. K., Blum H., Ohnishi T. The iron-sulphur centres of aerobically-grown Escherichia coli K12. FEBS Lett. 1980 Feb 25;111(1):223–227. doi: 10.1016/0014-5793(80)80798-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leigh J. S., Jr, Erecinska M. Thermodynamic and EPR characterization of mitochondrial succinate-cytochrome c reductase-phospholipid complexes. Biochim Biophys Acta. 1975 Apr 14;387(1):95–106. doi: 10.1016/0005-2728(75)90054-7. [DOI] [PubMed] [Google Scholar]
- Magnusson K., Hederstedt L., Rutberg L. Cloning and expression in Escherichia coli of sdhA, the structural gene for cytochrome b558 of the Bacillus subtilis succinate dehydrogenase complex. J Bacteriol. 1985 Jun;162(3):1180–1185. doi: 10.1128/jb.162.3.1180-1185.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson K., Philips M. K., Guest J. R., Rutberg L. Nucleotide sequence of the gene for cytochrome b558 of the Bacillus subtilis succinate dehydrogenase complex. J Bacteriol. 1986 Jun;166(3):1067–1071. doi: 10.1128/jb.166.3.1067-1071.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson K., Rutberg B., Hederstedt L., Rutberg L. Characterization of a pleiotropic succinate dehydrogenase-negative mutant of Bacillus subtilis. J Gen Microbiol. 1983 Apr;129(4):917–922. doi: 10.1099/00221287-129-4-917. [DOI] [PubMed] [Google Scholar]
- Malkin R., Vänngård T. An EPR study of cytochromes from spinach chloroplasts. FEBS Lett. 1980 Feb 25;111(1):228–231. doi: 10.1016/0014-5793(80)80799-x. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson N. R., Hansen R. E., Beinert H. EPR studies of the cytochrome b-c 1 segment of the mitochondrial electron transfer system. Biochem Biophys Res Commun. 1971 Nov;45(4):871–878. doi: 10.1016/0006-291x(71)90419-0. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson N. R., Hansen R. E., Beinert H. Electron paramagnetic resonance-detectable electron acceptors in beef heart mitochondria. Ubihydroquinone-cytochrome c reductase segment of the electron transfer system and complex mitochondrial fragments. J Biol Chem. 1974 Mar 25;249(6):1928–1939. [PubMed] [Google Scholar]
- Palmer G. The electron paramagnetic resonance of metalloproteins. Biochem Soc Trans. 1985 Jun;13(3):548–560. doi: 10.1042/bst0130548. [DOI] [PubMed] [Google Scholar]
- Salerno J. C. Cytochrome electron spin resonance line shapes, ligand fields, and components stoichiometry in ubiquinol-cytochrome c oxidoreductase. J Biol Chem. 1984 Feb 25;259(4):2331–2336. [PubMed] [Google Scholar]
- Saraste M. Location of haem-binding sites in the mitochondrial cytochrome b. FEBS Lett. 1984 Jan 30;166(2):367–372. doi: 10.1016/0014-5793(84)80114-3. [DOI] [PubMed] [Google Scholar]
- T'sai A. L., Palmer G. Purification and characterization of highly purified cytochrome b from complex III of baker's yeast. Biochim Biophys Acta. 1982 Sep 15;681(3):484–495. doi: 10.1016/0005-2728(82)90191-8. [DOI] [PubMed] [Google Scholar]
- Tochikubo K. Changes in terminal respiratory pathways of Bacillus subtilis during germination, outgrowth and vegetative growth. J Bacteriol. 1971 Nov;108(2):652–661. doi: 10.1128/jb.108.2.652-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widger W. R., Cramer W. A., Herrmann R. G., Trebst A. Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b6-f complex: position of the cytochrome b hemes in the membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):674–678. doi: 10.1073/pnas.81.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]


