Abstract
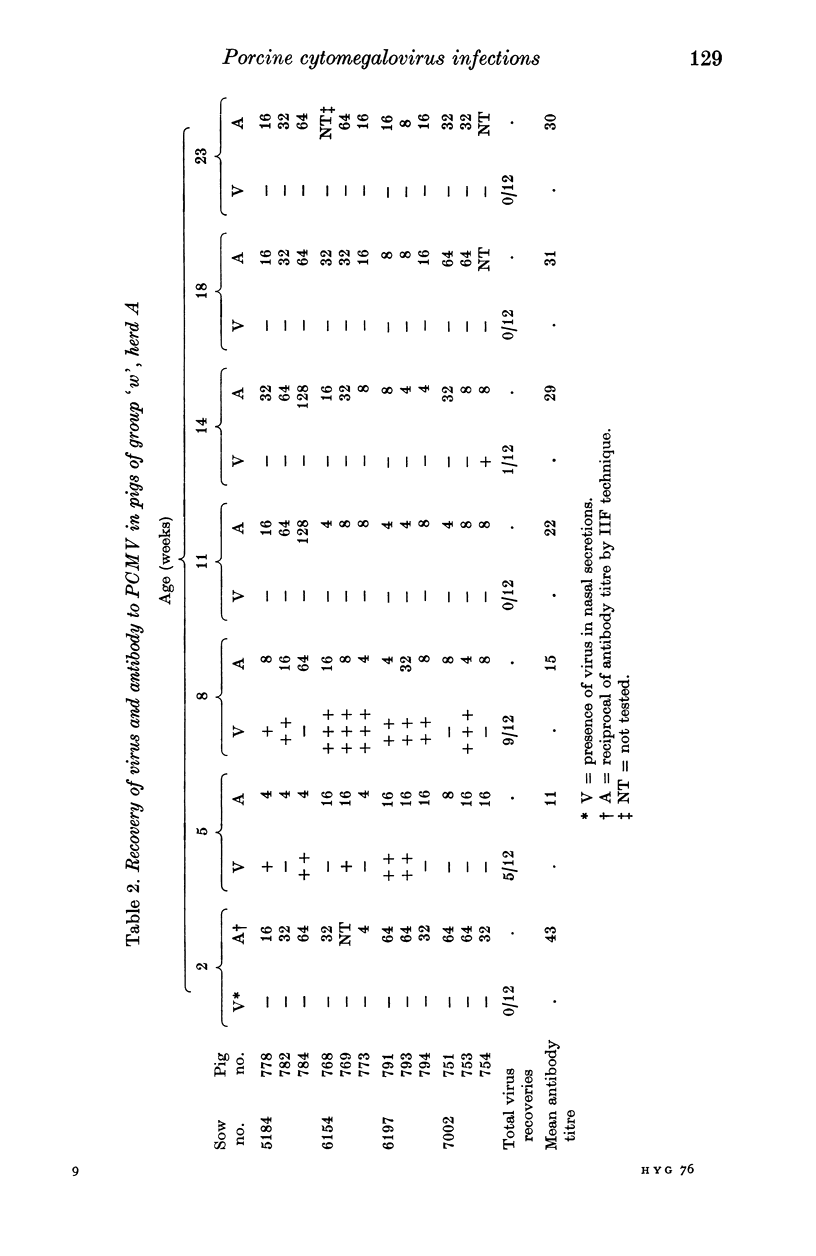
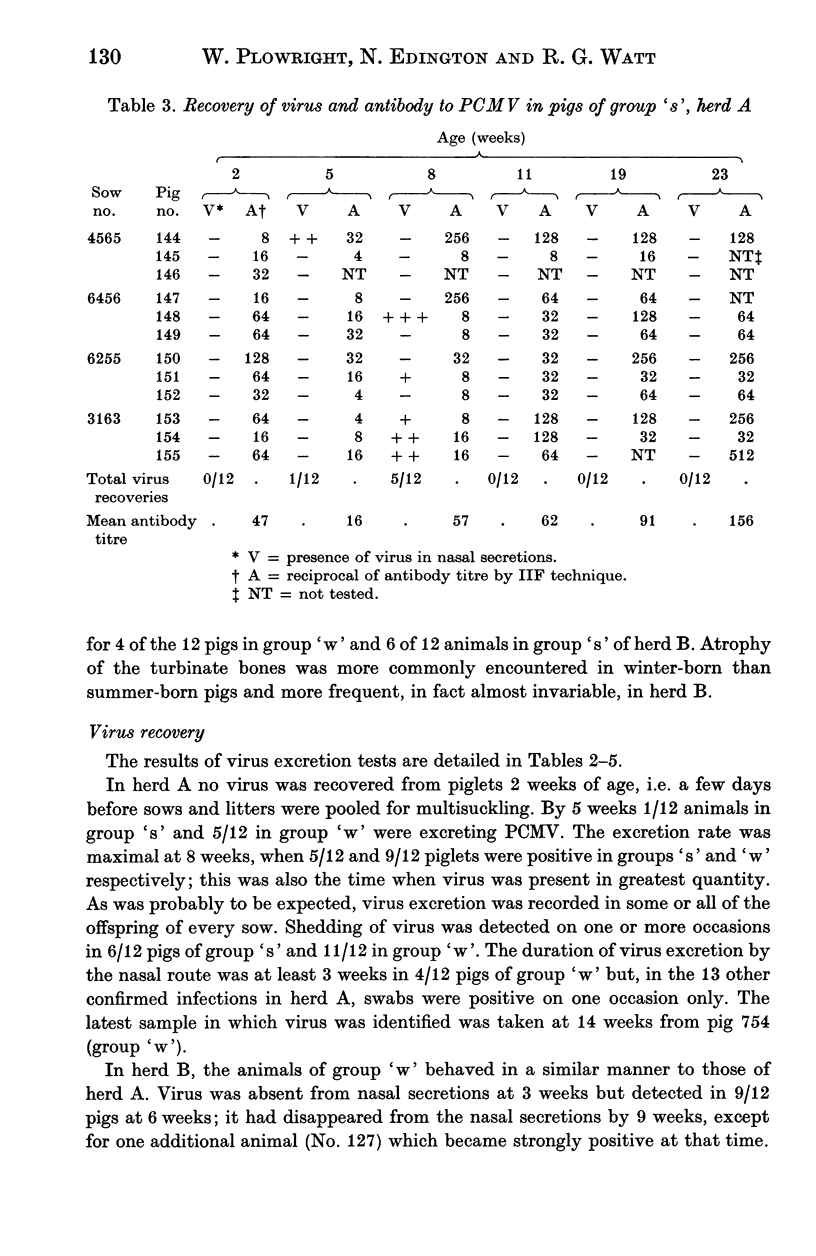
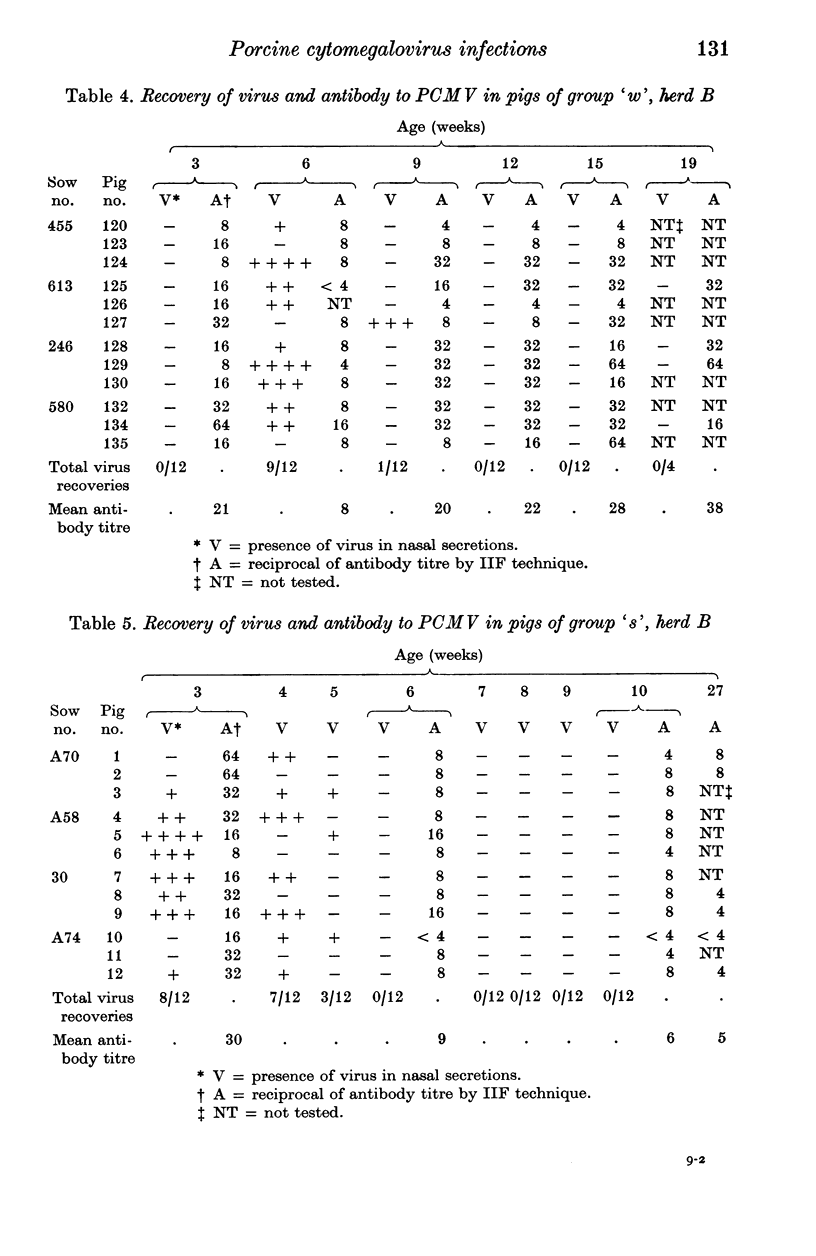
A longitudinal, virological and serological study of pigs in two herds with respiratory disease showed that infection by porcine cytomegalovirus (PCMV) was universal in both. Virus excretion usually began when piglets were between 3 and 6 weeks of age and reached a maximum between 5 and 8 weeks; it was usually no longer detectable at 11-12 weeks. Antibody demonstrable in indirect immunofluorescence (IIF) tests was present to moderate or high titre in all piglets at 2-3 weeks. This was presumed to be maternal in origin as it declined in titre between 2-3 and 5-6 weeks. After this fall the majority of piglets showed seroconversion as a result of virus infection. One group of 12 pigs in which infection occurred earlier than usual showed a very poor antibody response, which, nevertheless, persisted through to week 27. The findings are discussed with relation to porcine atrophic rhinitis and cytomegalovirus infection in other species.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENYESH-MELNICK M., ROSENBERG H. S., WATSON B. VIRUSES IN CELL CULTURES OF KIDNEYS OF CHILDREN WITH CONGENITAL HEART MALFORMATIONS AND OTHER DISEASES. Proc Soc Exp Biol Med. 1964 Nov;117:452–459. doi: 10.3181/00379727-117-29607. [DOI] [PubMed] [Google Scholar]
- BRODSKY I., ROWE W. P. Chronic subclinical infection with mouse salivary gland virus. Proc Soc Exp Biol Med. 1958 Dec;99(3):654–655. doi: 10.3181/00379727-99-24451. [DOI] [PubMed] [Google Scholar]
- Cox F., Hughes W. T. Fecal excretion of cytomegalovirus in disseminated cytomegalic inclusion disease. J Infect Dis. 1974 Jun;129(6):732–736. doi: 10.1093/infdis/129.6.732. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEDEARIS D. N., Jr MOUSE CYTOMEGALOVIRUS INFECTION. II. OBSERVATIONS DURING PROLONGED INFECTIONS. Am J Hyg. 1964 Jul;80:103–112. [PubMed] [Google Scholar]
- Plummer G. Cytomegaloviruses of man and animals. Prog Med Virol. 1973;15:92–125. [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., CRAMBLETT H. G., MASTROTA F. M. Detection of human salivary gland virus in the mouth and urine of children. Am J Hyg. 1958 Jan;67(1):57–65. doi: 10.1093/oxfordjournals.aje.a119919. [DOI] [PubMed] [Google Scholar]
- Starr J. G., Gold E. Prevalence and duration of postnatally acquired human cytomegalovirus infection. J Chronic Dis. 1970 Feb;22(8):603–607. doi: 10.1016/0021-9681(70)90036-6. [DOI] [PubMed] [Google Scholar]
- Stern H. Isolation of cytomegalovirus and clinical manifestations of infection at different ages. Br Med J. 1968 Mar 16;1(5593):665–669. doi: 10.1136/bmj.1.5593.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLER T. H., HANSHAW J. B. Virologic and clinical observations on cytomegalic inclusion disease. N Engl J Med. 1962 Jun 14;266:1233–1244. doi: 10.1056/NEJM196206142662401. [DOI] [PubMed] [Google Scholar]
- Watt R. G., Plowright W., Sabo A., Edington N. A sensitive cell culture system for the virus of porcine inclusion body rhinitis (cytomegalic inclusion disease). Res Vet Sci. 1973 Jan;14(1):119–121. [PubMed] [Google Scholar]