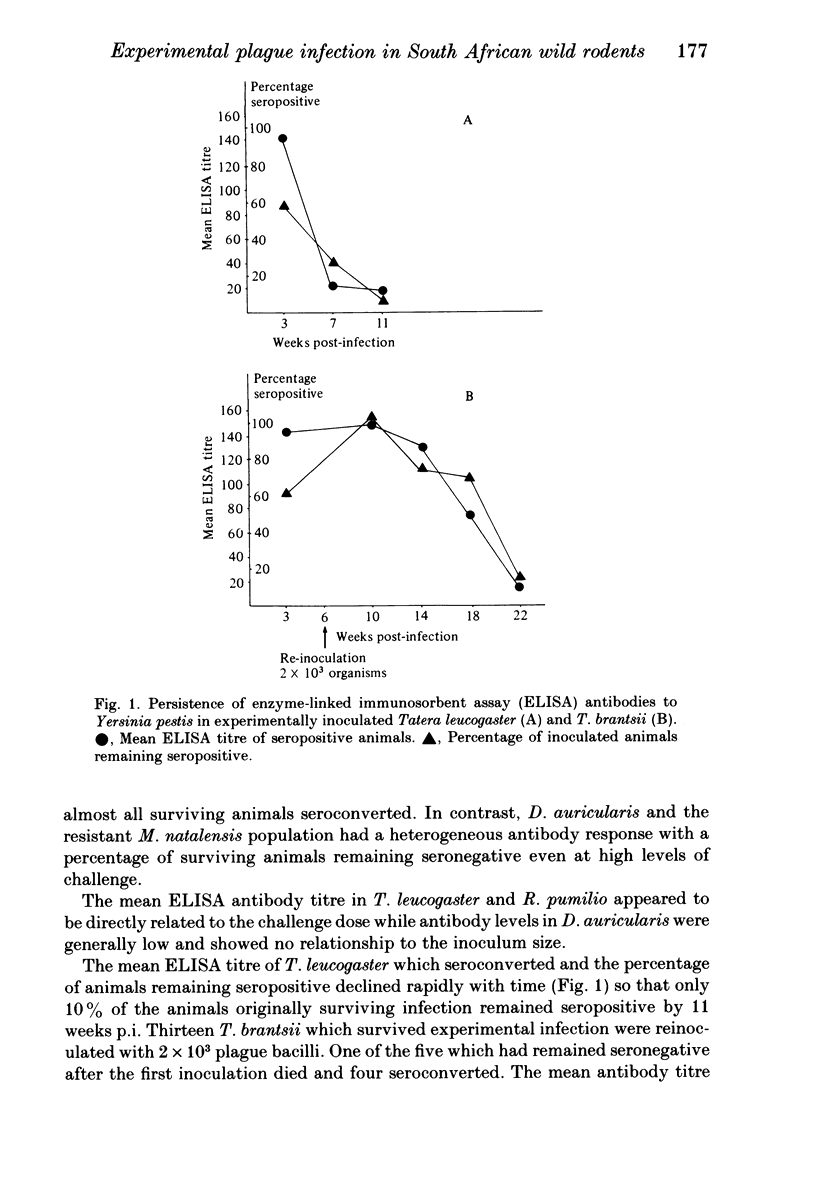
Abstract
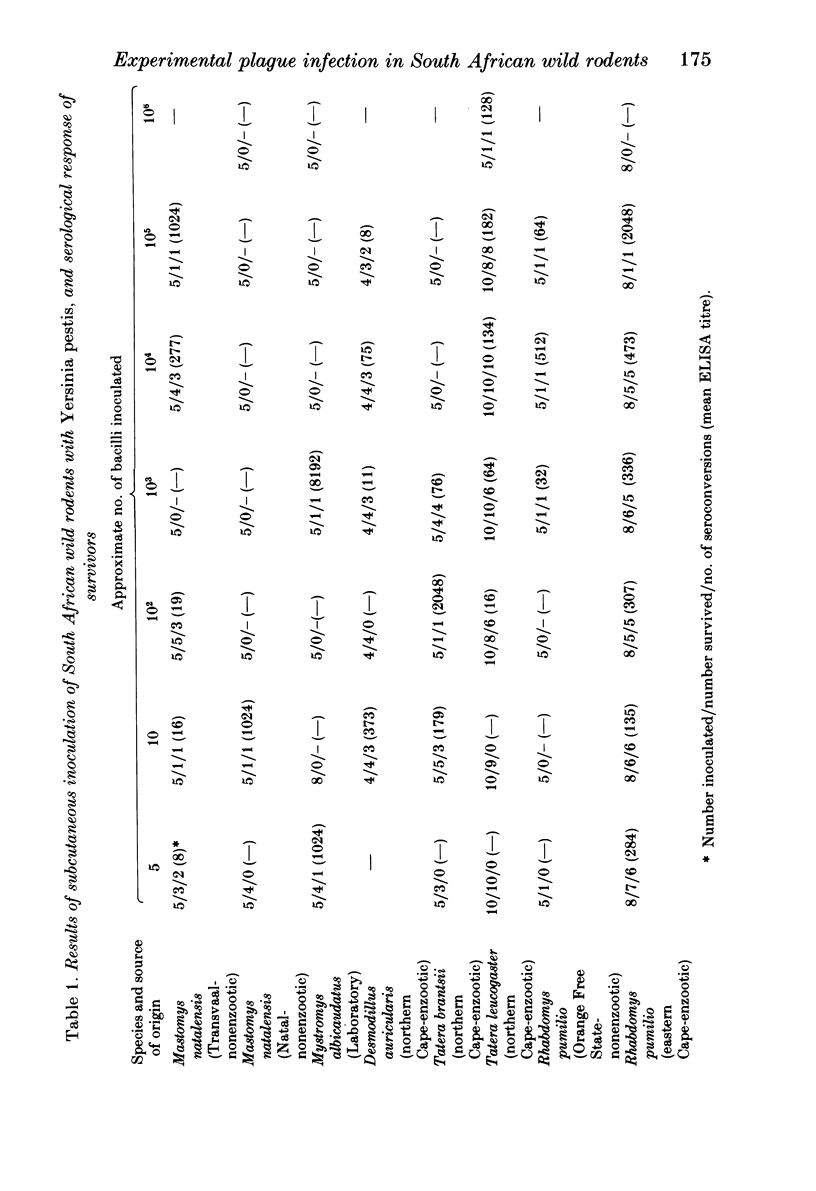
Susceptibility studies were undertaken to determine the response of some South African wild rodent species to experimental plague (Yersinia pestis) infection. A degree of plague resistance was found in three gerbil species captured in the plague enzootic region of the northern Cape Province, these being the Namaqua gerbil, Desmodillus auricularis, (LD50 1 X 10(6) organisms), the bushveld gerbil, Tatera leucogaster, (LD50 9.1 X 10(5)) and the highveld gerbil, T. brantsii (LD50 4 X 10(2)). Animals from a population of the four-striped mouse, Rhabdomys pumilio, captured in the plague area of Port Elizabeth, proved moderately resistant to experimental plague infection (LD50 1.3 X 10(4)) while those from another population of the same species captured in a plague-free area of the Orange Free State were extremely susceptible (LD50, 5 organisms). The response of both populations however was a heterogeneous one. Marked differences in susceptibility were also found between two populations of multimammate mice, Mastomys natalensis (2n = 32) although both originated from areas outwith the known distribution of plague in southern Africa. The 50% infectious dose was relatively high in T. leucogaster (3.2 X 10(2)) and D. auricularis (1.7 X 10(3)), but was low (2-16 organisms) in the other rodent species tested. The plague antibody response, determined by enzyme-linked immunosorbent assay (ELISA), was extremely short-lived in T. leucogaster, only 10% of inoculated animals remaining seropositive at low titres after 11 weeks. Antibodies persisted for only slightly longer in the sera of T. brantsii which were reinoculated with 2 X 10(3) plague organisms 6 weeks after initial challenge. The demonstration of the existence of both susceptible and resistant populations of R. pumilio and M. natalensis indicates that these species must be considered as potential plague reservoir hosts in parts of South Africa. The results suggest that resistance to plague infection in previously epizootic hosts in the northern Cape Province such as Tatera sp. and D. auricularis has arisen through continual selective pressure of the organism. If the findings are applicable to gerbil populations in other plague enzootic regions of South Africa it is probable that acquired plague resistance has been responsible for the absence of gerbil epizootics and consequently for the dramatic decline in human plague outbreaks in South Africa since 1950.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALTAZARD M., BAHMANYAR M., MOSTACHFI P., EFTEKHARI M., MOFIDI C. [Research on plague in Iran]. Bull World Health Organ. 1960;23:141–155. [PMC free article] [PubMed] [Google Scholar]
- DAVIS D. H. Plague in Africa from 1935 to 1949; a survey of wild rodents in African territories. Bull World Health Organ. 1953;9(5):665–700. [PMC free article] [PubMed] [Google Scholar]
- DAVIS D. H. Plague in South Africa: a study of the epizootic cycle in gerbils (Tatera brantsi) in the northern Orange Free State. J Hyg (Lond) 1953 Dec;51(4):427–449. doi: 10.1017/s0022172400036718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDENBERG M. I., QUAN S. F., HUDSON B. W. THE DETECTION OF INAPPARENT INFECTIONS WITH PASTEURELLA PESTIS IN A MICROTUS CALIFORNICUS POPULATION IN THE SAN FRANCISCO BAY AREA. Zoonoses Res. 1964 Apr;3:1–13. [PubMed] [Google Scholar]
- Green C. A., Gordon D. H., Lyons N. F. Biological species in Praomys (Mastomys) natalensis (Smith), a rodent carrier of Lassa virus and bubonic plague in Africa. Am J Trop Med Hyg. 1978 May;27(3):627–629. doi: 10.4269/ajtmh.1978.27.627. [DOI] [PubMed] [Google Scholar]
- Hallett A. F., McNeill D., Meyer K. F. A serological survey of the small mammals for plague in southern Africa. S Afr Med J. 1970 Jul 18;44(29):831–837. [PubMed] [Google Scholar]
- Hubbert W. T., Goldenberg M. I. Natural resistance to plague: genetic basis in the vole (Microtus californicus). Am J Trop Med Hyg. 1970 Nov;19(6):1015–1019. doi: 10.4269/ajtmh.1970.19.1015. [DOI] [PubMed] [Google Scholar]
- Isaäcson M., Taylor P., Arntzen L. Ecology of plague in Africa: response of indigenous wild rodents to experimental plague infection. Bull World Health Organ. 1983;61(2):339–344. [PMC free article] [PubMed] [Google Scholar]
- MOODY M. D., WINTER C. C. Rapid identification of Pasteurella pestis with fluorescent antibody. III. Staining Pasteurella pestis in tissue impression smears. J Infect Dis. 1959 May-Jun;104(3):288–294. doi: 10.1093/infdis/104.3.288. [DOI] [PubMed] [Google Scholar]
- QUAN S. F., KARTMAN L. Ecological studies of wild rodent plague in the San Francisco Bay Area of California. VIII. Susceptibility of wild rodents to experimental plague infection. Zoonoses Res. 1962 Jan 5;1:121–144. [PubMed] [Google Scholar]
- Shepherd A. J., Hummitzsch D. E., Leman P. A., Hartwig E. K. Studies on plague in the eastern Cape Province of South Africa. Trans R Soc Trop Med Hyg. 1983;77(6):800–808. doi: 10.1016/0035-9203(83)90293-6. [DOI] [PubMed] [Google Scholar]
- Shepherd A. J., Leman P. A., Hummitzsch D. E., Swanepoel R. A comparison of serological techniques for plague surveillance. Trans R Soc Trop Med Hyg. 1984;78(6):771–773. doi: 10.1016/0035-9203(84)90014-2. [DOI] [PubMed] [Google Scholar]
- Shepherd A. J., Leman P. A. Plague in South African rodents 1972-1981. Trans R Soc Trop Med Hyg. 1983;77(2):208–211. doi: 10.1016/0035-9203(83)90072-x. [DOI] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D., Albizo J. M. Practical applications of new laboratory methods for plague investigations. Bull World Health Organ. 1970;42(6):993–997. [PMC free article] [PubMed] [Google Scholar]
- Williams J. E., Harrison D. N., Cavanaugh D. C. Letter: Cryptic infection of rats with non-encapsulated variants of Yersinia pestis. Trans R Soc Trop Med Hyg. 1975;69(1):171–172. doi: 10.1016/0035-9203(75)90039-5. [DOI] [PubMed] [Google Scholar]
- Williams J. E., Moussa M. A., Cavanaugh D. C. Experimental plague in the California ground squirrel. J Infect Dis. 1979 Oct;140(4):618–621. doi: 10.1093/infdis/140.4.618. [DOI] [PubMed] [Google Scholar]


