Abstract
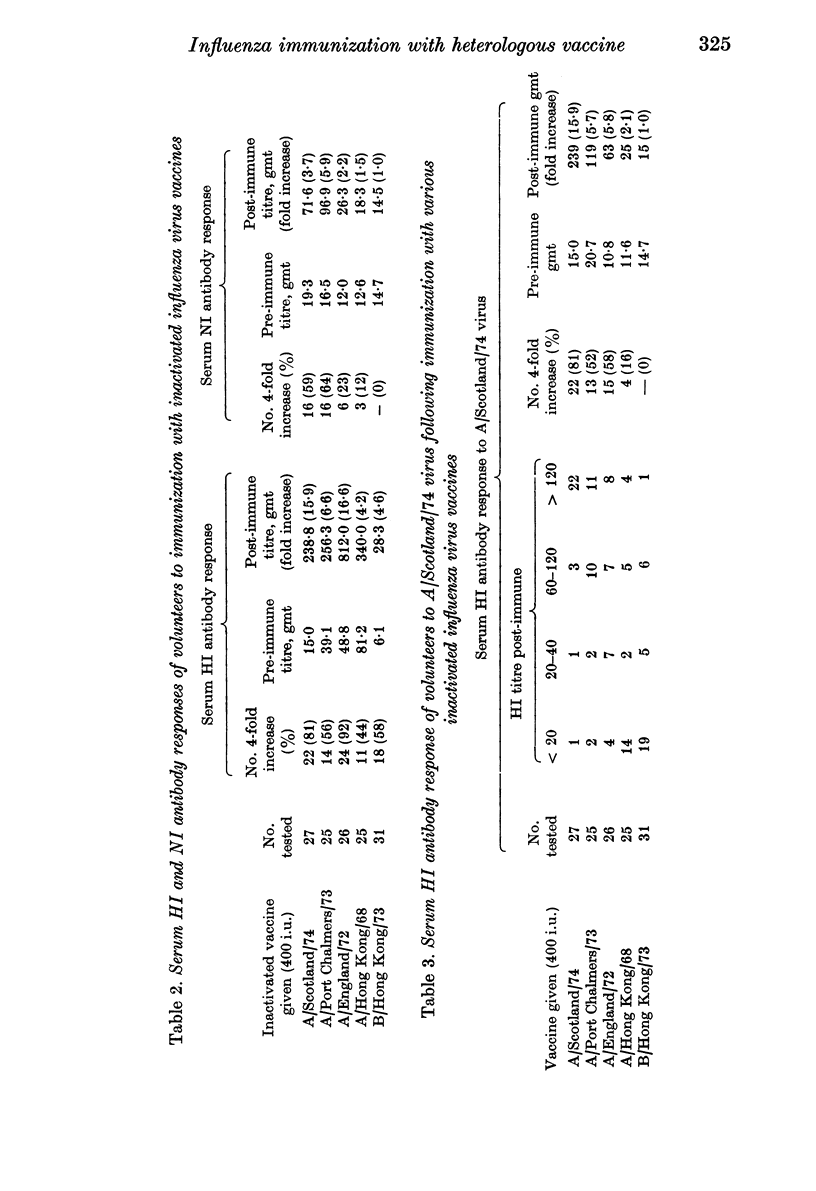
Groups of student volunteers were immunized with one of five different inactivated influenza virus vaccines. The concentration of virus in the various vaccines differed by both the international unitage test and by the concentration of haemagglutinin, as measured by the single radial diffusion test; the results of the two methods of standardization showed no correlation. The serum HI response to immunization was variable; volunteers given A/England/72 showed a 16·6-fold increase in homologous serum antibody titre whilst volunteers given A/Hong Kong/68 vaccine showed a 4·2-fold increase. The variable response of volunteers to immunization could not be explained by the varied concentration of virus in the vaccines, as measured by either test, the titres of serum HI antibody present before immunization, or a combination of these two factors.
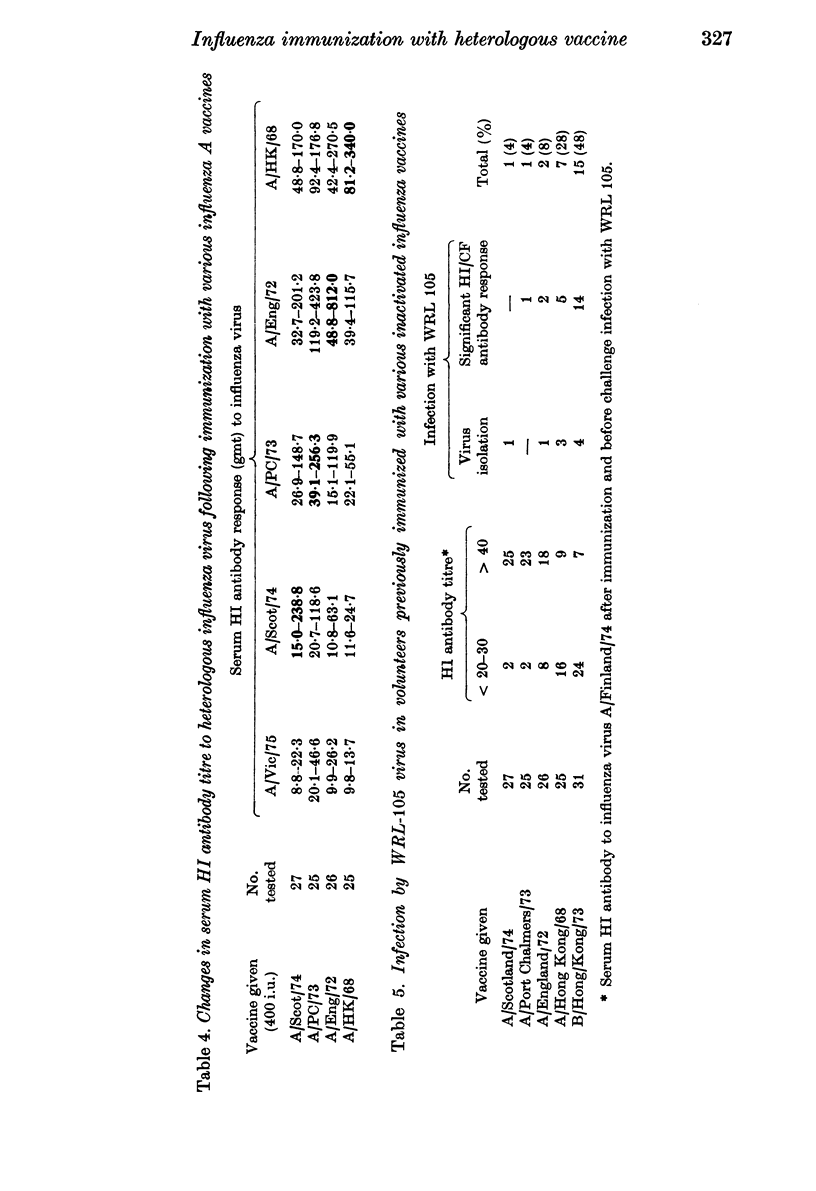
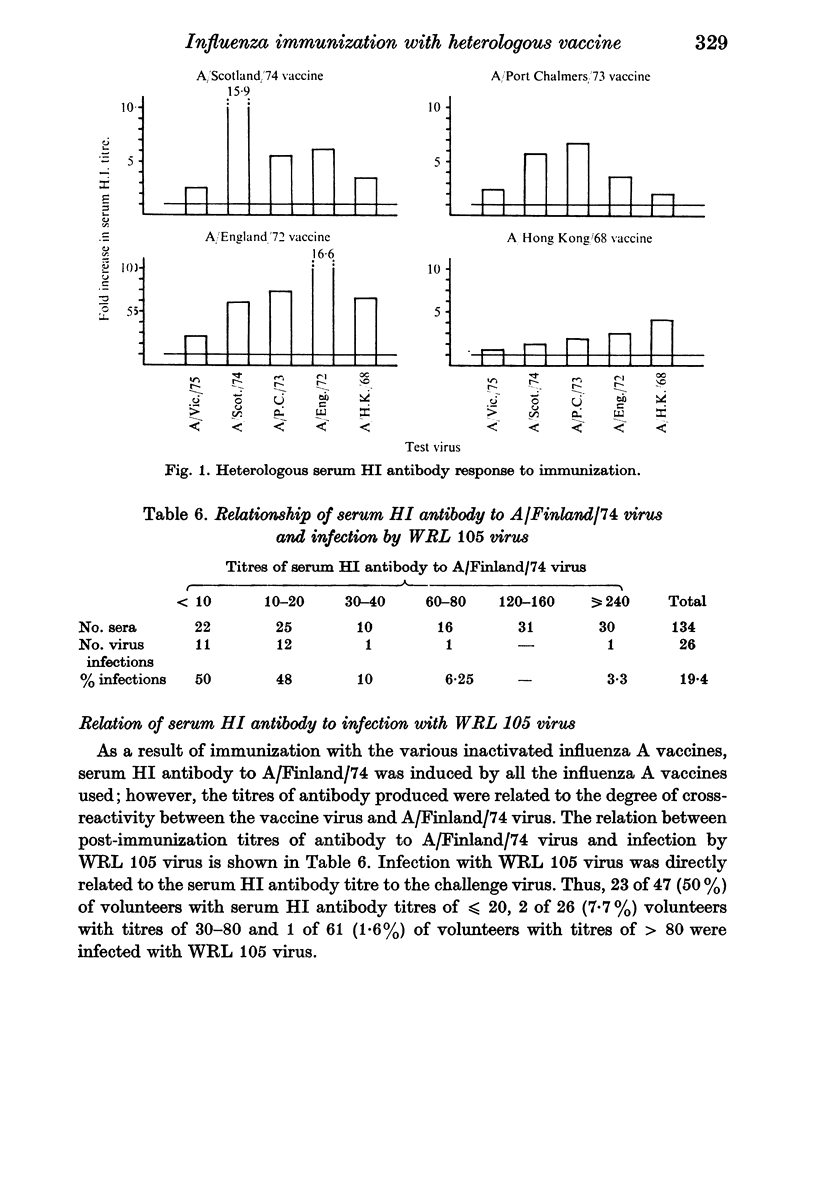
The ability to infect volunteers with WRL 105 virus 4 weeks after immunization with heterologous, inactivated virus vaccine was directly related to the degree of cross-reactivity between the haemagglutinins of this vaccine virus and WRL 105 virus. Thus, the greatest number of infections by the challenge virus were seen in volunteers given A/Hong Kong/68 vaccine, less were observed in volunteers given A/England/72 vaccine, and least were found in groups given A/Port Chalmers/73 or A/Scotland/74 vaccine. However, compared with the incidence of infection in volunteers given B/Hong Kong/73 vaccine, all the heterologous influenza A vaccine gave some immunity to challenge infection.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- BRADSTREET C. M., TAYLOR C. E. Technique of complementfixation test applicable to the diagnosis of virus diseases. Mon Bull Minist Health Public Health Lab Serv. 1962 May;21:96–104. [PubMed] [Google Scholar]
- Beare A. S., Hobson D., Reed S. E., Tyrrell D. A. A comparison of live and killed influenza-virus vaccines. Report to the Medical Research Council's Committee on Influenza and other Respiratory Virus Vaccines. Lancet. 1968 Aug 24;2(7565):418–422. doi: 10.1016/s0140-6736(68)90463-7. [DOI] [PubMed] [Google Scholar]
- Bevan A. M., Furminger I. G., Smith C. H. Neuraminidase assay of influenza vaccines. Dev Biol Stand. 1975;28:173–180. [PubMed] [Google Scholar]
- Foy H. M., Cooney M. K., McMahan R. A Hong Kong influenza immunity three years after immunization. JAMA. 1973 Nov 12;226(7):758–761. [PubMed] [Google Scholar]
- Foy H. M., Cooney M. K., McMahan R., Bor E., Grayston J. T. Single-dose monovalent A 2 -Hong Kong influenza vaccine. Efficacy 14 months after immunization. JAMA. 1971 Aug 23;217(8):1067–1071. [PubMed] [Google Scholar]
- Freestone D. S., Bowker C. H., Letley E., Ferris R. D., White W. G., Barnes G. M. A clinical trial of WRL 105 strain live attenuated influenza vaccine comparing four methods of intranasal vaccination. J Hyg (Lond) 1976 Jun;76(3):459–466. doi: 10.1017/s002217240005539x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson D. Assessment of the efficacy of influenza vaccines against natural challenge. Dev Biol Stand. 1975;28:285–294. [PubMed] [Google Scholar]
- Hoskins T. W., Davies J. R., Allchin A., Miller C. L., Pollock T. M. Controlled trial of inactivated influenza vaccine containing the a-Hong Kong strain during an outbreak of influenza due to the a-England-42-72 strain. Lancet. 1973 Jul 21;2(7821):116–120. doi: 10.1016/s0140-6736(73)93062-6. [DOI] [PubMed] [Google Scholar]
- MEIKLEJOHN G., KEMPE C. H., THALMAN W. G., LENNETTE E. H. Evaluation of monovalent influenza vaccines. II. Observations during an influenza a-prime epidemic. Am J Hyg. 1952 Jan;55(1):12–21. doi: 10.1093/oxfordjournals.aje.a119500. [DOI] [PubMed] [Google Scholar]
- Morris C. A., Freestone D. S., Stealey V. M., Oliver P. R. Recombinant WRL 105 strain live attenuated influenza vaccine. Immunogenicity, reactivity, and transmissibility. Lancet. 1975 Aug 2;2(7927):196–199. doi: 10.1016/s0140-6736(75)90670-4. [DOI] [PubMed] [Google Scholar]
- Pereira M. S., Chakraverty P., Schild G. C., Coleman M. T., Dowdle W. R. Prevalence of antibody to current influenza viruses and effect of vaccination on antibody response. Br Med J. 1972 Dec 23;4(5842):701–703. doi: 10.1136/bmj.4.5842.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., Jennings R., McLaren C., Clarke A. Immune response in volunteers to intranasal inoculation with freeze-dried influenza Q/Hong Kong/68 vaccine. J Biol Stand. 1975;3(1):41–50. doi: 10.1016/0092-1157(75)90006-2. [DOI] [PubMed] [Google Scholar]
- Potter C. W., Jennings R., McLaren C., Edey D., Stuart-Harris C. H., Brady M. A new surface-antigen-adsorbed influenza virus vaccine. II. Studies in a volunteer group. J Hyg (Lond) 1975 Dec;75(3):353–362. doi: 10.1017/s0022172400024414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., Jennings R., Phair J. P., Clarke A., Stuart-Harris C. H. Dose-response relationship after immunization of volunteers with a new, surface-antigen-adsorbed influenza virus vaccine. J Infect Dis. 1977 Mar;135(3):423–431. doi: 10.1093/infdis/135.3.423. [DOI] [PubMed] [Google Scholar]
- Potter C. W., Jennings R. The hamster as a model system for the study of influenza vaccines. Postgrad Med J. 1976 Jun;52(608):345–351. doi: 10.1136/pgmj.52.608.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben F. L., Johnston F., Streiff E. J. Influenza in a partially immunized aged population. Effectiveness of killed Hong Kong vaccine against infection with the England strain. JAMA. 1974 Nov 11;230(6):863–866. doi: 10.1001/jama.230.6.863. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Schild G. C., Oxford J. S., Dowdle W. R., Coleman M., Pereira M. S., Chakraverty P. Antigenic variation in current influenza A viruses: evidence for a high frequency of antigenic 'drift' for the Hong Kong virus. Bull World Health Organ. 1974;51(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- Schild G. C., Wood J. M., Newman R. W. A single-radial-immunodiffusion technique for the assay of influenza haemagglutinin antigen. Proposals for an assay method for the haemagglutinin content of influenza vaccines. Bull World Health Organ. 1975;52(2):223–231. [PMC free article] [PubMed] [Google Scholar]
- Stiver H. G., Graves P., Eickhoff T. C., Meiklejohn G. Efficacy of "Hong Kong" vaccine in preventing "England" variant influenza A in 1972. N Engl J Med. 1973 Dec 13;289(24):1267–1271. doi: 10.1056/NEJM197312132892402. [DOI] [PubMed] [Google Scholar]


