Abstract
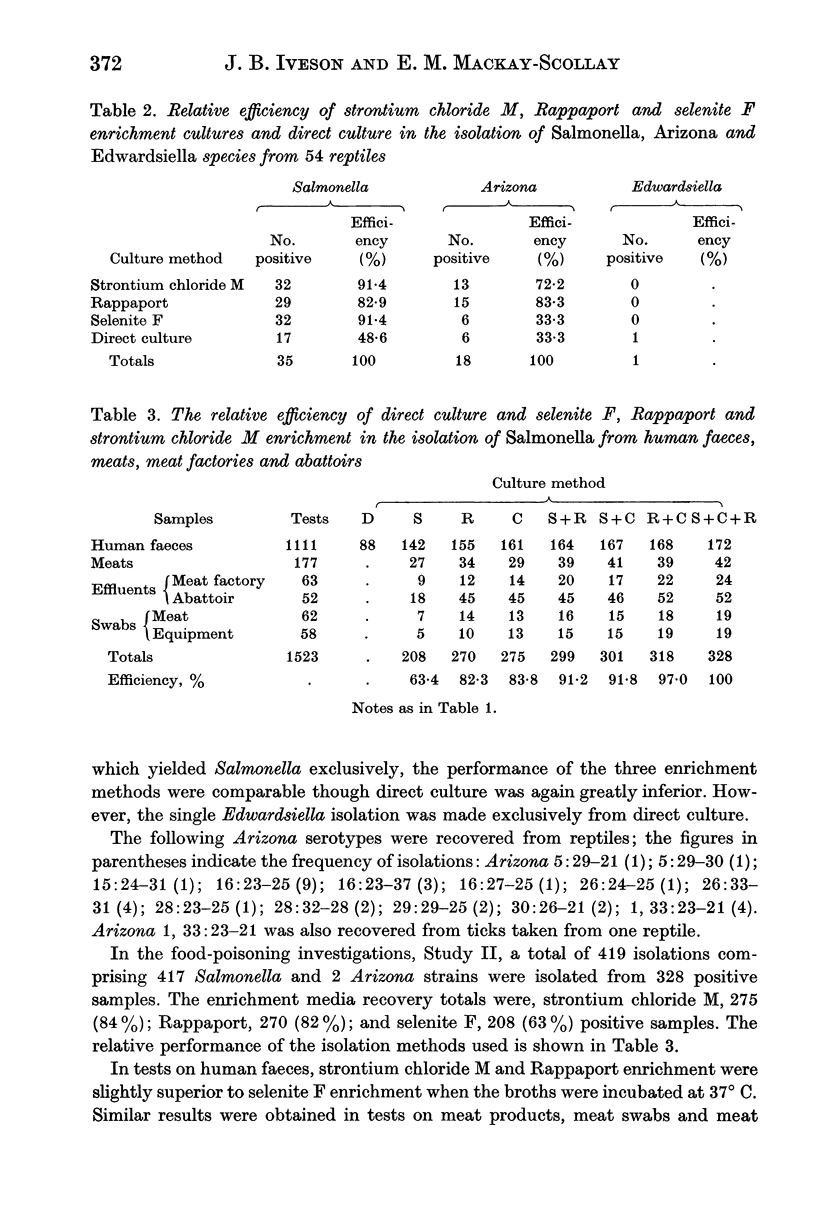
Strontium chloride enrichment broth was found to be comparable to Rappaport broth for the recovery of a wide range of Salmonella serotypes from man, animals, meat products and effluents. With the exception of cloacal samples from reptiles, both procedures were superior to selenite F.
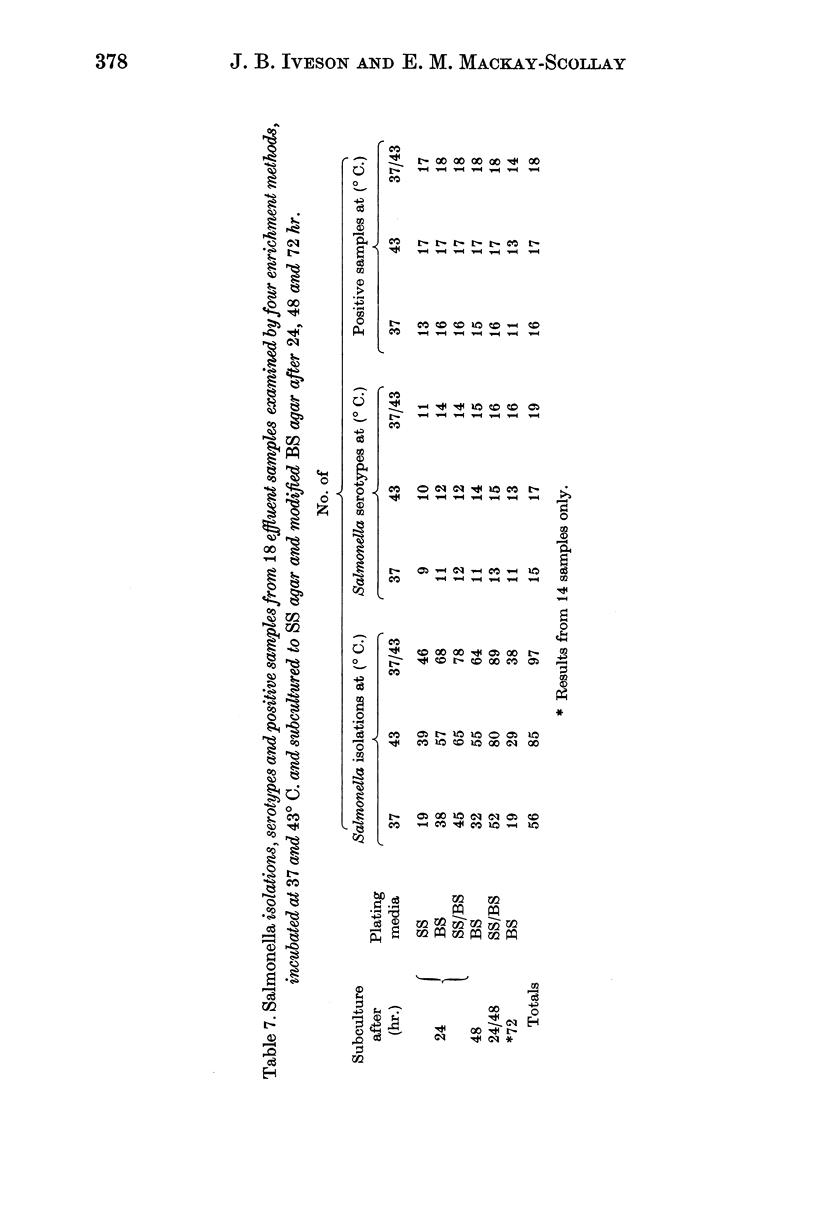
The performance of strontium chloride Mand selenite F enrichment was improved when effluent samples were incubated at 43° C.
Strontium chloride M and Rappaport enrichment were superior to selenite F for the isolation of Arizona species from reptiles.
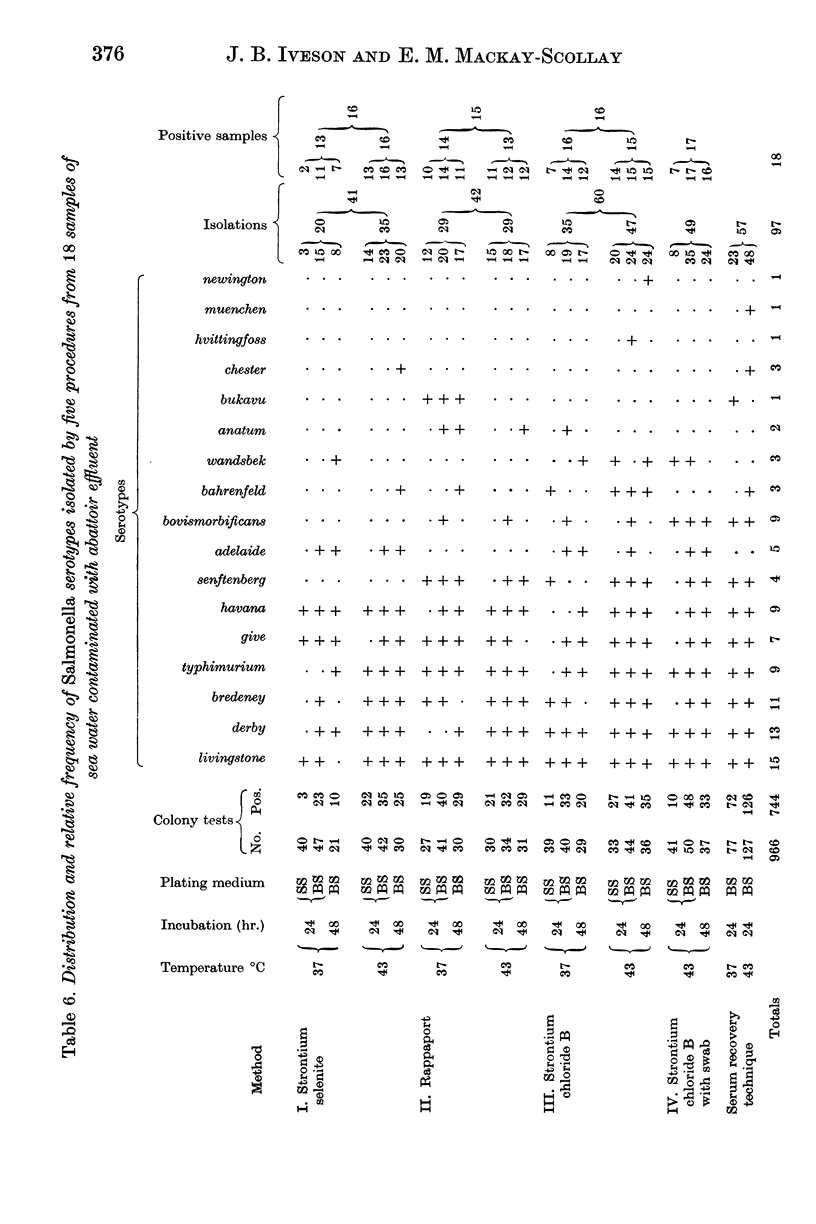
Strontium chloride B, strontium selenite and Rappaport broths were found suitable for the isolation of multiple Salmonella serotypes from sea water contaminated with abattoir effluents. The strontium chloride B and strontium selenite enrichment media were superior to Rappaport broth when samples were incubated at 43° C.
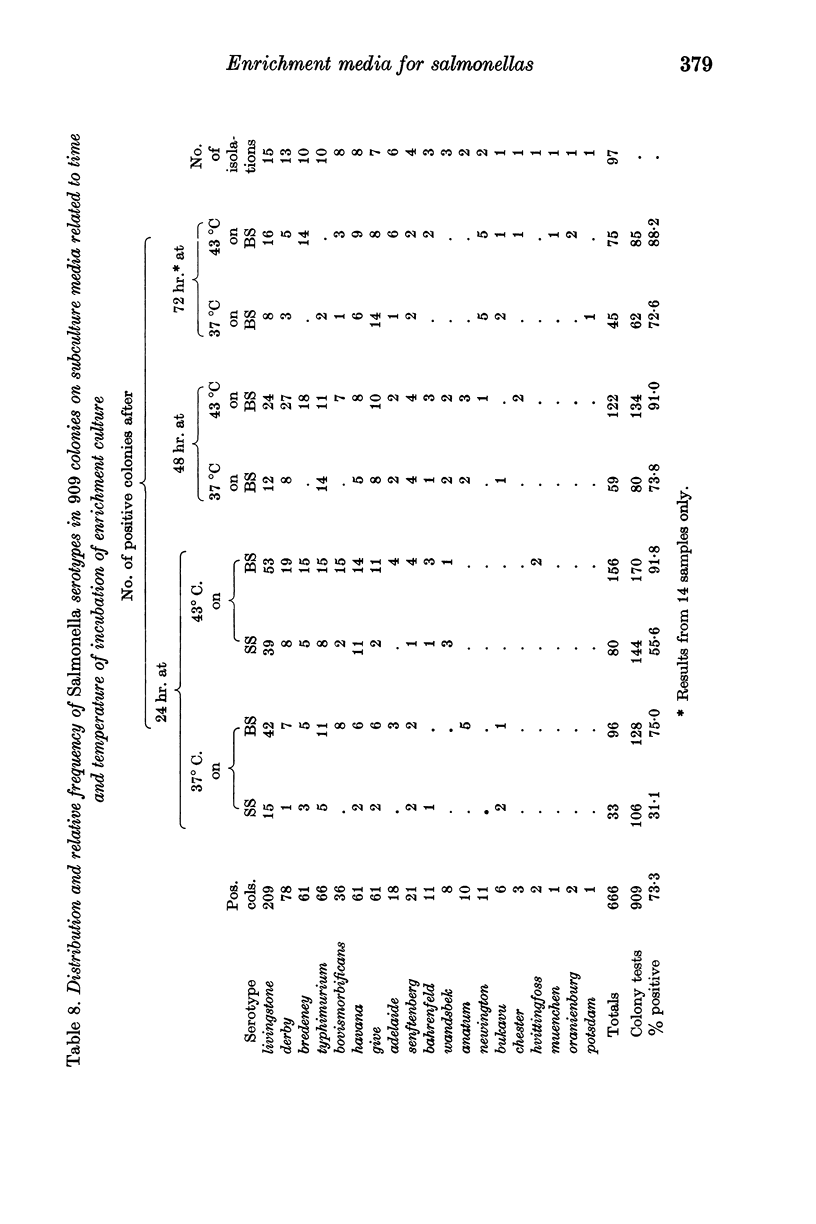
Modified bismuth sulphite agar was found superior to Salmonella—Shigella agar as a solid subculture medium.
The investigation of a food poisoning outbreak due to Salmonella typhimurium phage type 21 is reported.
The significance of the choice of sampling and isolation techniques in salmonellosis in man and animals is discussed.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K., Kennedy H. Comparison of selective media for the isolation of salmonellae. J Clin Pathol. 1965 Nov;18(6):747–749. doi: 10.1136/jcp.18.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beh K. J. The incidence of enteropathogenic Escherichia coli serotypes and Salmonella SPP in pigs in New South Wales. Aust Vet J. 1971 Aug;47(8):379–382. doi: 10.1111/j.1751-0813.1971.tb09216.x. [DOI] [PubMed] [Google Scholar]
- COLLARD P., UNWIN M. A trial of Rappaport's medium. J Clin Pathol. 1958 Sep;11(5):426–427. doi: 10.1136/jcp.11.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUICKSHANK J. C., SMITH H. W. Isolation of salmonellae from dogs, cats, and pigeons. Br Med J. 1949 Dec 3;2(4639):1254–1258. doi: 10.1136/bmj.2.4639.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edel W., Kampelmacher E. H. Comparative studies on Salmonella-isolation in eight European laboratories. Bull World Health Organ. 1968;39(3):487–491. [PMC free article] [PubMed] [Google Scholar]
- Edel W., Kampelmacher E. H. Salmonella isolation in nine European laboratories using a standardized technique. Bull World Health Organ. 1969;41(2):297–306. [PMC free article] [PubMed] [Google Scholar]
- GALTON M. M., SCATTERDAY J. E., HARDY A. V. Salmonellosis in dogs. I. Bacteriological, epidemiological and clinical considerations. J Infect Dis. 1952 Jul-Aug;91(1):1–5. doi: 10.1093/infdis/91.1.1. [DOI] [PubMed] [Google Scholar]
- GALTON M. M., SMITH W. V., McELRATH H. B., HARDY A. B. Salmonella in swine, cattle and the environment of abattoirs. J Infect Dis. 1954 Nov-Dec;95(3):236–245. doi: 10.1093/infdis/95.3.236. [DOI] [PubMed] [Google Scholar]
- HARVEY R. W., PHILLIPS W. P. An environmental survey of bakehouses and abattoirs for salmonellae. J Hyg (Lond) 1961 Mar;59:93–103. doi: 10.1017/s0022172400038730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R. W., THOMSON S. Optimum temperature of incubation for isolation of Salmonellae. Mon Bull Minist Health Public Health Lab Serv. 1953 Jul;12:149–150. [PubMed] [Google Scholar]
- Harvey R. W., Price T. H. Elevated temperature incubation of enrichment media for the isolation of salmonellas from heavily contaminated materials. J Hyg (Lond) 1968 Sep;66(3):377–381. doi: 10.1017/s0022172400041243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. W., Price T. H. Sewer and drain swabbing as a means of investigating salmonellosis. J Hyg (Lond) 1970 Dec;68(4):611–624. doi: 10.1017/s0022172400042546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. W., Price T. H. The examination of samples infected with multiple salmonella serotypes. J Hyg (Lond) 1967 Sep;65(3):423–434. doi: 10.1017/s0022172400045939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper W. L., Jenkins H. R. An evaluation of Rappaport's magnesium chloride/malachite green medium in the routine examination of faeces. J Hyg (Lond) 1965 Dec;63(4):491–495. doi: 10.1017/s002217240004537x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss M. Studies on Salt Action: VI. The Stimulating and Inhibitive Effect of Certain Cations upon Bacterial Growth. J Bacteriol. 1923 Mar;8(2):141–162. doi: 10.1128/jb.8.2.141-162.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVESON J. B., KOVACS N., LAURIE W. AN IMPROVED METHOD OF ISOLATING SALMONELLAE FROM CONTAMINATED DESICCATED COCONUT. J Clin Pathol. 1964 Jan;17:75–78. doi: 10.1136/jcp.17.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iveson J. B., Kovacs N. A comparative trial of Rappaport enrichment medium for the isolation of Salmonellae from faeces. J Clin Pathol. 1967 May;20(3):290–293. doi: 10.1136/jcp.20.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iveson J. B., Mackay-Scollay E. M., Bamford V. Salmonella and Arizona in reptiles and man in Western Australia. J Hyg (Lond) 1969 Mar;67(1):135–145. doi: 10.1017/s0022172400041516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iveson J. B., Mackay-Scollay E. M. Strontium chloride and strontium selenite enrichment broth media in the isolation of Salmonella. J Hyg (Lond) 1969 Sep;67(3):457–464. doi: 10.1017/s0022172400041875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iveson J. B. Strontium chloride B and E.E. enrichment broth media for the isolation of Edwardsiella, Salmonella and Arizona species from tiger snakes. J Hyg (Lond) 1971 Sep;69(3):323–330. doi: 10.1017/s0022172400021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONFORTI N., NAVON B., RAPPAPORT F. A new enrichment medium for certain Salmonellae. J Clin Pathol. 1956 Aug;9(3):261–266. doi: 10.1136/jcp.9.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE P. E., MACKERRAS I. M. Salmonella infections of Australian native animals. Aust J Exp Biol Med Sci. 1955 Feb;33(1):117–125. doi: 10.1038/icb.1955.13. [DOI] [PubMed] [Google Scholar]
- McDONAGH V. P., SMITH H. G. The significance of the abattoir in salmonella infection in Bradford. J Hyg (Lond) 1958 Jun;56(2):271–279. doi: 10.1017/s002217240003775x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M. G. The incidence of Salmonella in normal slaughtered pigs. Aust Vet J. 1970 Feb;46(2):40–43. doi: 10.1111/j.1751-0813.1970.tb05027.x. [DOI] [PubMed] [Google Scholar]
- SMITH H. W., BUXTON A. Isolation of Salmonellae from faeces of domestic animals. Br Med J. 1951 Jun 30;1(4721):1478–1483. doi: 10.1136/bmj.1.4721.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliadis P. Shigella spp., Salmonella choleraesuis and Arizona in Rappaport's medium. J Appl Bacteriol. 1968 Sep;31(3):367–372. doi: 10.1111/j.1365-2672.1968.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Vassiliadis P., Trichopoulos D., Papadakis J., Politi G. Salmonella isolations in abattoirs in Greece. J Hyg (Lond) 1970 Dec;68(4):601–609. doi: 10.1017/s0022172400042534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schothorst M., Kampelmacher E. H. Salmonella in meat imported from South American countries. J Hyg (Lond) 1967 Sep;65(3):321–325. doi: 10.1017/s0022172400045848. [DOI] [PMC free article] [PubMed] [Google Scholar]


