Abstract
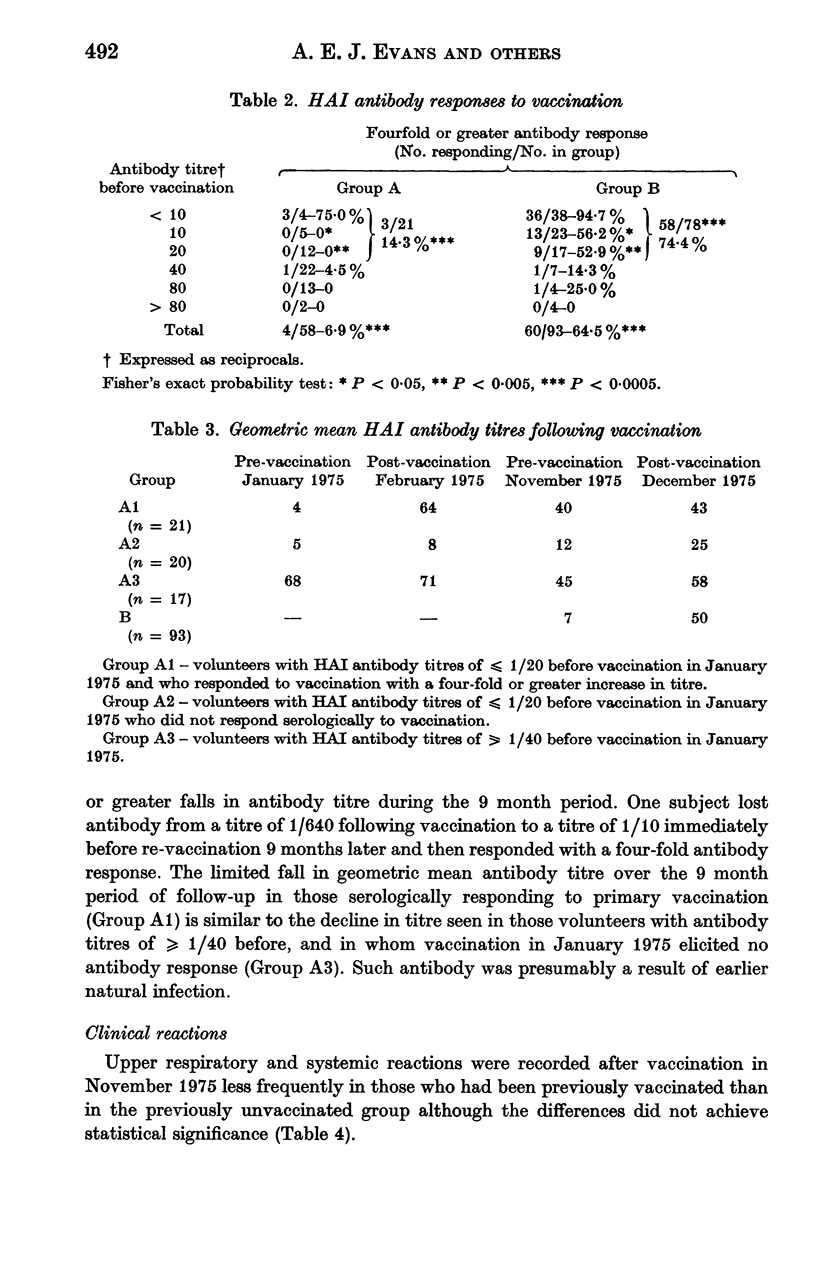
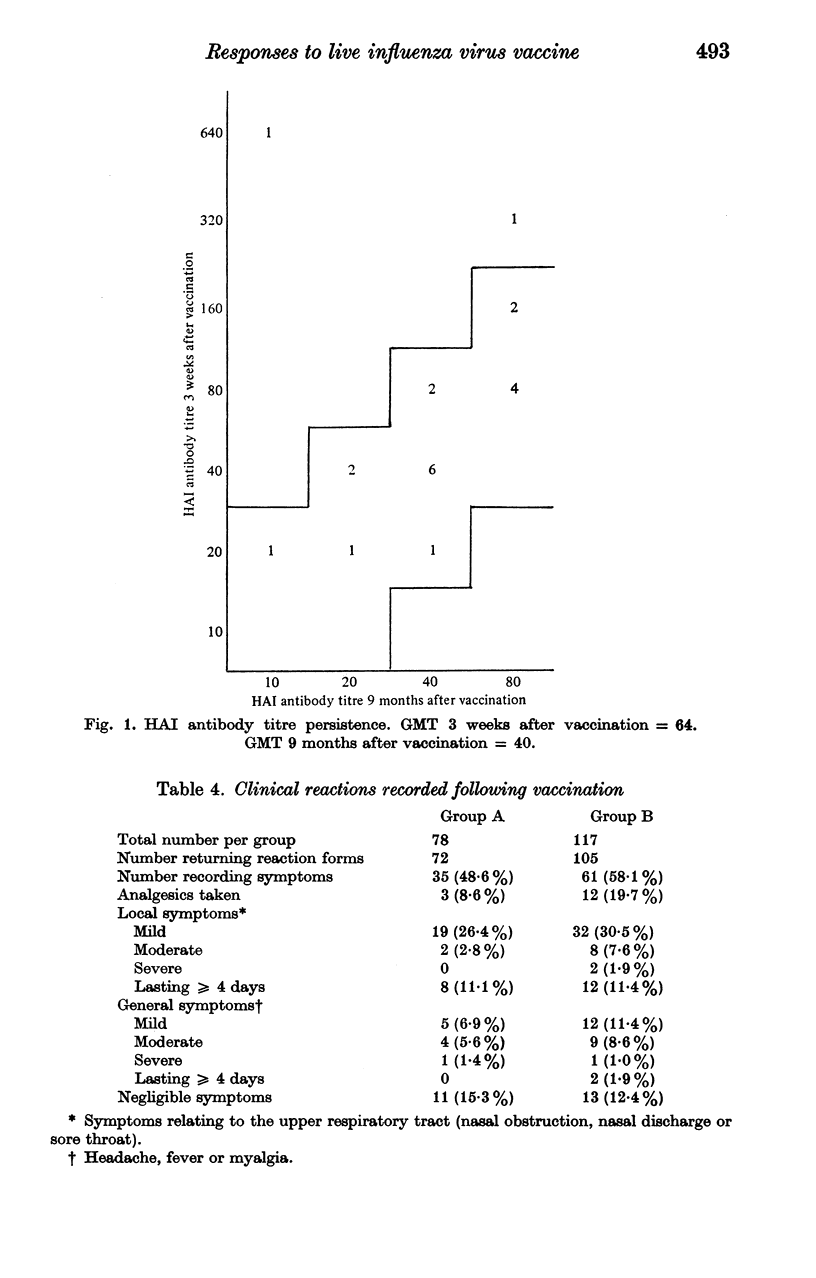
Intranasal vaccination with a single 0.5 ml dose of 10(7.0) EID 50 WRL 105 strain live influenza vaccine elicited four-fold or greater increases in circulating homotypic haemagglutinating inhibiting (HAI) antibody in 60 (64.5%) of 93 volunteers, or in 58 (74.4%) of 78 volunteers with HAI antibody titres before vaccination of less than or equal to 1/20. In comparison, in a group of volunteers vaccinated 9 months previously re-vaccination elicited antibody responses in only 4 (6.9%) of 58 volunteers, or in 3 (14.3%) of 21 volunteers with antibody titres before vaccination of less than or equal to 1/20. Titres of vaccine-induced antibody and antibody resulting from earlier natural infection appeared to fall slowly and at equivalent rates over a 9 month period.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans A. E., Letley E., Ferris R. D., Freestone D. S. WRL 105 strain live attenuated influenza vaccine; comparison of one and two dose schedules. J Hyg (Lond) 1976 Dec;77(3):327–332. doi: 10.1017/s0022172400055686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton R. J., Jennings R., Potter C. W. Differential response of ferrets to infection with virulent and avirulent influenza viruses: a possible marker of virus attenuation. Arch Virol. 1977;55(1-2):55–66. doi: 10.1007/BF01314479. [DOI] [PubMed] [Google Scholar]
- Freestone D. S., Bowker C. H., Letley E., Ferris R. D., White W. G., Barnes G. M. A clinical trial of WRL 105 strain live attenuated influenza vaccine comparing four methods of intranasal vaccination. J Hyg (Lond) 1976 Jun;76(3):459–466. doi: 10.1017/s002217240005539x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone D. S. Clinical trials with intranasally administered WRL 105 strain live influenza vaccine in volunteers. Dev Biol Stand. 1976;33:207–212. [PubMed] [Google Scholar]
- Freestone D. S., Hamilton-Smith S., Schild G. C., Buckland R., Chinn S., Tyrrell D. A. Antibody responses and resistance to challenge in volunteers vaccinated with live attenuated, detergent split and oil adjuvant A2-Hong Kong-68 (H 3 N 2 ) influenza vaccines. A report to the Medical Research Council Committee on Influenza and other Respiratory Virus Vaccines. J Hyg (Lond) 1972 Sep;70(3):531–543. doi: 10.1017/s0022172400063117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEIKLEJOHN G., KEMPE C. H., THALMAN W. G., LENNETTE E. H. Evaluation of monovalent influenza vaccines. II. Observations during an influenza a-prime epidemic. Am J Hyg. 1952 Jan;55(1):12–21. doi: 10.1093/oxfordjournals.aje.a119500. [DOI] [PubMed] [Google Scholar]
- Moffat M. A., Stealey V. M., Freestone D. S., Macdonald A. Assessment of elicited antibody responses, clinical reactions and transmissibility of WRL 105 live influenza vaccine. J Biol Stand. 1976 Apr;4(2):91–95. doi: 10.1016/0092-1157(76)90017-2. [DOI] [PubMed] [Google Scholar]
- Morris C. A., Freestone D. S., Stealey V. M., Oliver P. R. Recombinant WRL 105 strain live attenuated influenza vaccine. Immunogenicity, reactivity, and transmissibility. Lancet. 1975 Aug 2;2(7927):196–199. doi: 10.1016/s0140-6736(75)90670-4. [DOI] [PubMed] [Google Scholar]
- Mostow S. R., Tyrrell D. A. Detection of attenuation of recombinant influenza viruses in vitro. Lancet. 1972 Jul 15;2(7768):116–117. doi: 10.1016/s0140-6736(72)91599-1. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Stealey V. M., McCahon D., Freestone D. S. Preparation and characterization of live recombinant influenza vaccine. Dev Biol Stand. 1976;33:191–196. [PubMed] [Google Scholar]


