Abstract
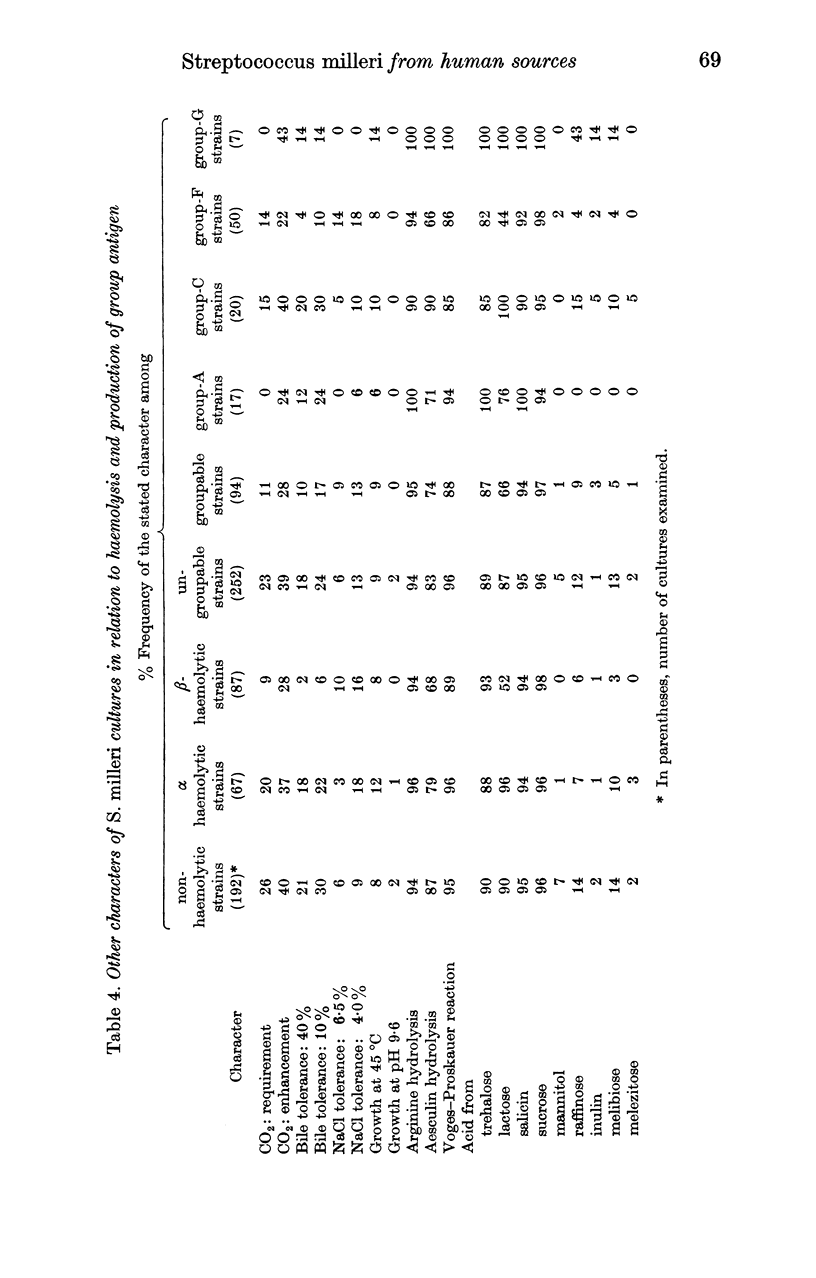
A collection of 346 strains of Streptococcus milleri from a variety of human sources was examined culturally and biochemically, and for the presence of Lancefield group antigens. Most of the strains were non-haemolytic and ungroupable, but 25% were β haemolytic and 19% were α haemolytic; 28% possessed a group antigen (A, 5%; C, 6%, F, 14%, G, 3%). These antigens were present in 69% of β-haemolytic but in only 13% of α-haemolytic or non-haemolytic strains; β haemolysis occurred in 82% of group-F strains, 43% of other groupable strains and 11% of ungroupable strains.
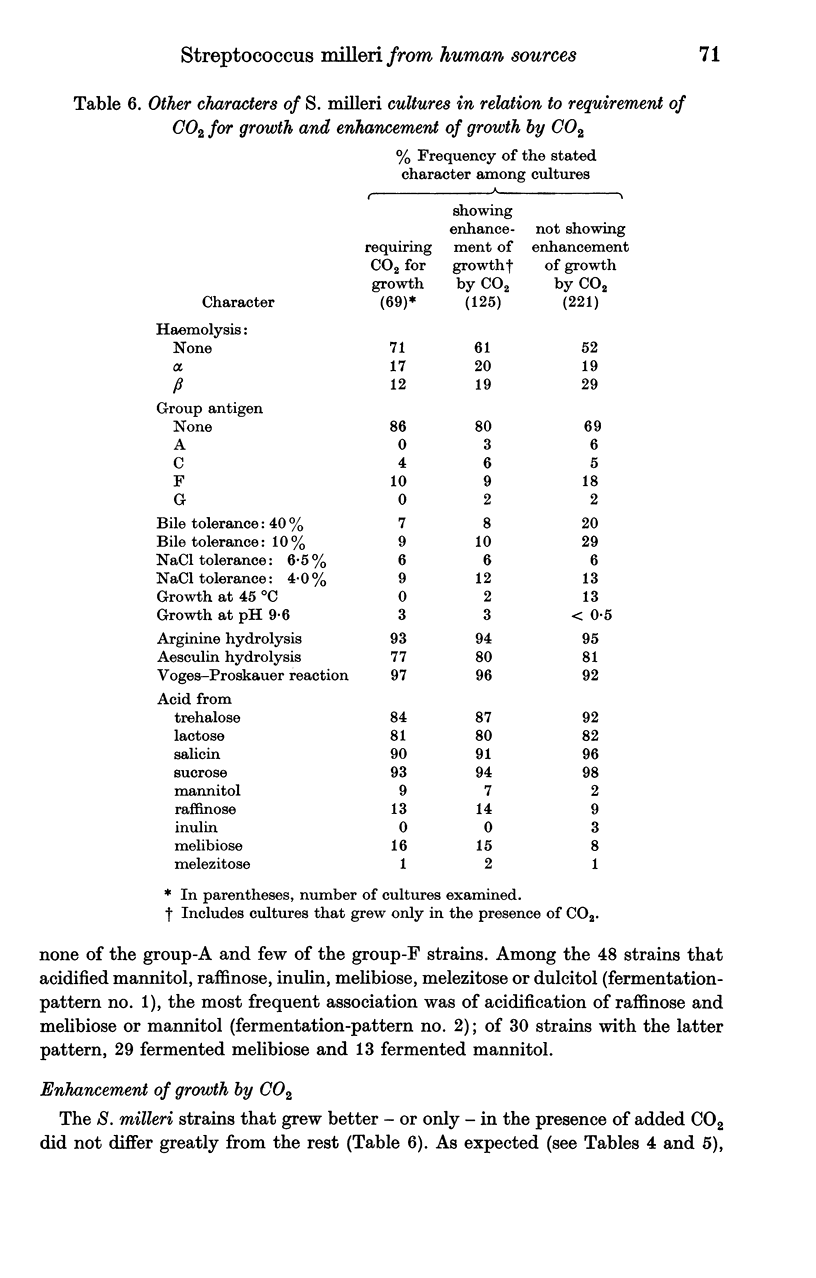
The following reactions were given by > 80% of S. milleri strains: hydrolysis of arginine and aesculin, a positive Voges—Proskauer reaction, and acidification of trehalose, lactose, salicin and sucrose. A minority of strains showed enhancement of growth by CO2, bile tolerance, NaCl tolerance, and ability to acidify other sugars, notably mannitol, raffinose and melibiose. Departures from the modal pattern of biochemical reactions showed a weak correlation with the type of haemolysis and the presence or absence of a group antigen but were not sufficiently systematic for clear-cut subdivisions to be recognized within the species.
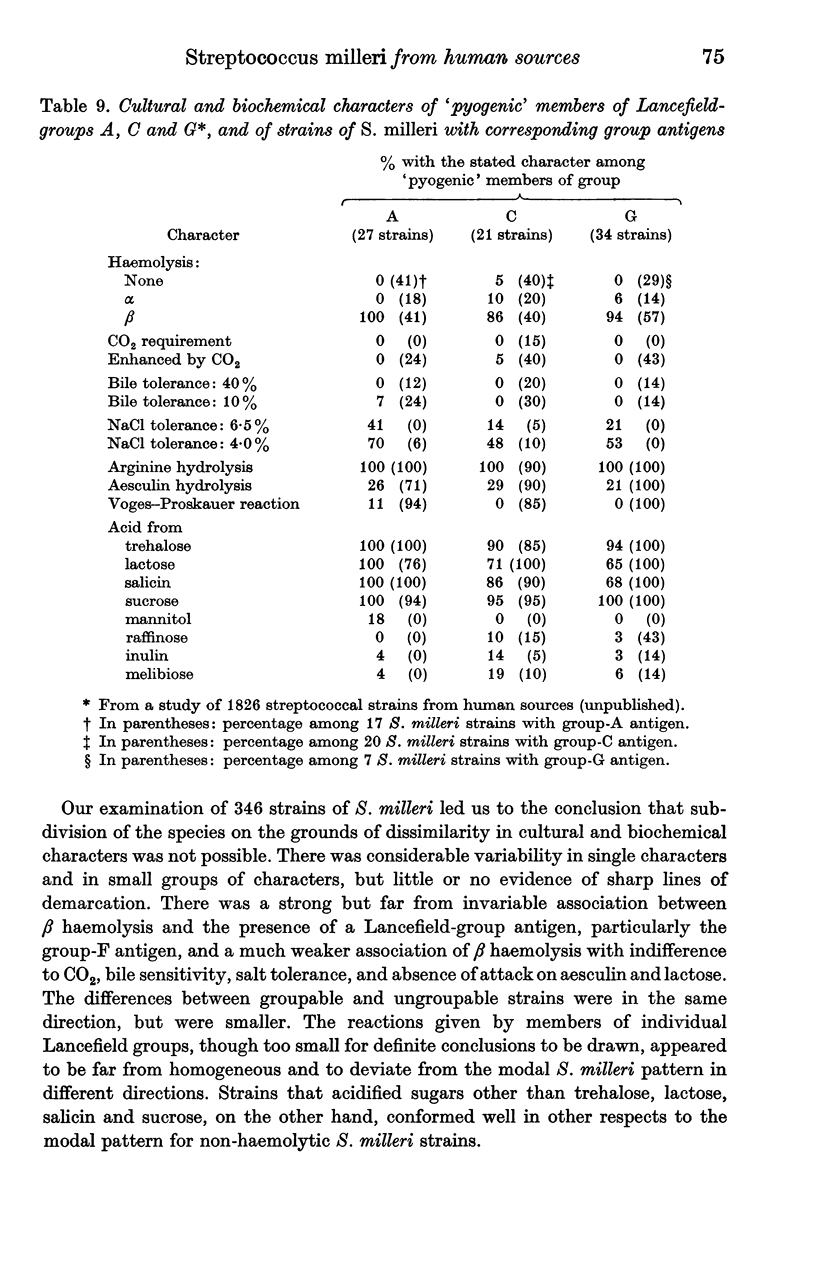
S. milleri therefore appeared to comprise a `central' group of non-haemolytic strains that rarely formed a Lancefield-group antigen, in which the aesculin reaction was nearly always positive, lactose was usually acidified, and a considerable minority showed enhancement of growth by CO2 and bile tolerance. Deviations from this pattern were of two main types. (1) `Loss' of one or more of these reactions, which tended to be associated with β-haemolysis and presence of a group antigen. In these respects, α-haemolytic strains tended to occupy an intermediate position. (2) `Gain' of the ability to acidify additional sugars, notably raffinose and melibiose or mannitol; this occurred mainly among otherwise typical non-haemolytic strains that were rarely groupable.
Only 12% of isolations from the bloodstream of patients suffering from systemic infections were β haemolytic and only 18% possessed a group antigen, but a considerably greater proportion of those from visceral abscesses were β haemolytic (28%). Among isolations from superficial lesions in some body sites there were considerably greater proportions of β-haemolytic and groupable strains; thus, nearly one-half of those isolated from the abdomen other than the female genital tract were β-haemolytic and over one-half were groupable. On the other hand, strains from the teeth and gums were nearly always non-haemolytic and ungroupable, and most vaginal isolations were of non-haemolytic strains with a wide sugar-fermentation pattern.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bliss E. A. Studies upon Minute Hemolytic Streptococci: III. Serological Differentiation. J Bacteriol. 1937 Jun;33(6):625–642. doi: 10.1128/jb.33.6.625-642.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman G. The application of computers to the classification of streptococci. J Gen Microbiol. 1968 Jan;50(1):149–158. doi: 10.1099/00221287-50-1-149. [DOI] [PubMed] [Google Scholar]
- Colman G., Williams R. E. The cell walls of streptococci. J Gen Microbiol. 1965 Dec;41(3):375–387. doi: 10.1099/00221287-41-3-375. [DOI] [PubMed] [Google Scholar]
- DEIBEL R. H., NIVEN C. F., Jr Reciprocal replacement of oleic acid and CO2 in the nutrition of the minute streptococci and Lactobacillus leichmannii. J Bacteriol. 1955 Aug;70(2):134–140. doi: 10.1128/jb.70.2.134-140.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTHOF O. Ueber pathogene vergrünende Streptokokken; Streptokokken-Befunde bei dentogenen Abszessen und Infiltraten im Bereich der Mundhöhle. Zentralbl Bakteriol Orig. 1956 Sep;166(7-8):553–564. [PubMed] [Google Scholar]
- LIU P. Carbon dioxide requirement of group F and minute colony G hemolytic streptococci. J Bacteriol. 1954 Sep;68(3):282–288. doi: 10.1128/jb.68.3.282-288.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejàre B., Edwardsson S. Streptococcus milleri (Guthof); an indigenous organism of the human oral cavity. Arch Oral Biol. 1975 Nov;20(11):757–762. doi: 10.1016/0003-9969(75)90048-5. [DOI] [PubMed] [Google Scholar]
- OTTENS H., WINKLER K. C. Indifferent and haemolytic streptococci possessing group-antigen F. J Gen Microbiol. 1962 Apr;28:181–191. doi: 10.1099/00221287-28-1-181. [DOI] [PubMed] [Google Scholar]
- Parker M. T., Ball L. C. Streptococci and aerococci associated with systemic infection in man. J Med Microbiol. 1976 Aug;9(3):275–302. doi: 10.1099/00222615-9-3-275. [DOI] [PubMed] [Google Scholar]
- Phillips I., Warren C., Harrison J. M., Sharples P., Ball L. C., Parker M. T. Antibiotic susceptibilities of streptococci from the mouth and blood of patients treated with penicillin or lincomycin and clindamycin. J Med Microbiol. 1976 Nov;9(4):393–404. doi: 10.1099/00222615-9-4-393. [DOI] [PubMed] [Google Scholar]
- Poole P. M., Wilson G. Streptococcus milleri in the appendix. J Clin Pathol. 1977 Oct;30(10):937–942. doi: 10.1136/jcp.30.10.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson J. P., Maxted W. R., Pinney A. M. An M-associated protein antigen (MAP) of group A streptococci. J Hyg (Lond) 1971 Dec;69(4):553–564. doi: 10.1017/s0022172400021823. [DOI] [PMC free article] [PubMed] [Google Scholar]


