Abstract
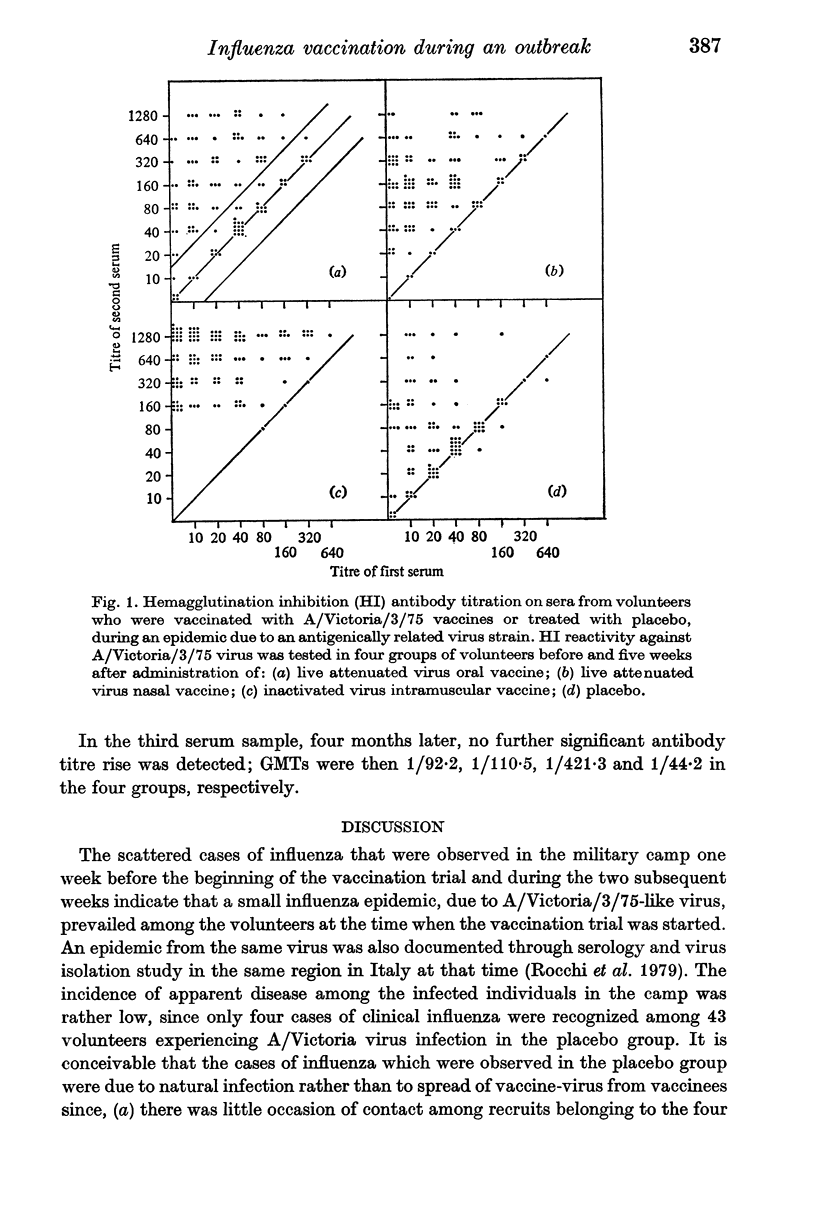
Immunization procedures with live attenuated and inactivated vaccines were carried out on a group of young recruits at the beginning of an outbreak of infection due to an A/Victoria/3/75-related virus strain, which occurred in February 1977 in a military camp. A retrospective investigation on protection from clinical influenza was then performed in order to investigate whether immunization with live virus vaccines, administered at the beginning of an epidemic, could provide early protection from the disease. In the course of the two weeks following vaccination, laboratory-confirmed clinical influenza cases occurred in 4 subjects among the 110 volunteers of the control group which received placebo, and in 8, 7 and 4 subjects respectively of the 3 groups of about 125 individuals, each of which received one of the following vaccine preparations: (a), live attenuated A/Victoria/3/75 influenza virus oral vaccine, grown on chick embryo kidney culture; (b), live attenuated nasal vaccine, a recombinant of A/Puerto Rico/8/34 with A/Victoria/3/75 virus; and (c), inactivated A/Victoria/3/75 virus intramuscular vaccine. These data do not support the hypothesis that, during an epidemic of infection, early protection from clinical influenza can be achieved through immunization with live attenuated or inactivated influenza virus vaccines, in spite of the high immunizing capability of the vaccine preparations.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleksandrova G. I., Smorodintsev A. A., Beljaeva N. M., Vasil'ev B. J., Geft R. A., Panteleev V. G., Sejfer M. A., Selivanov A. A. Testing the safety and effectiveness of oral administration of a live influenza vaccine. Bull World Health Organ. 1970;42(3):429–436. [PMC free article] [PubMed] [Google Scholar]
- Crifò S., Filiaci F., Pelosio A., Rocchi G. Risposta secretoria e sierica dopo vaccinazione antinfluenza le per via nasale con virus vivo attenuato A/Victoria/3/75. Boll Soc Ital Biol Sper. 1978 Jun 15;54(11):1021–1026. [PubMed] [Google Scholar]
- De Barbieri A., Crovari P., Cuneo-Crovari P., Giacometti G. Further researches on live influenza virus vaccines. Dev Biol Stand. 1977 Jun 1;39:73–76. [PubMed] [Google Scholar]
- Donikian M. A., McKee J., Greene L. C. Challenge versus natural infection as an index or protection after influenza immunization. Dev Biol Stand. 1977 Jun 1;39:149–154. [PubMed] [Google Scholar]
- Elveback L. R., Fox J. P., Ackerman E., Langworthy A., Boyd M., Gatewood L. An influenza simulation model for immunization studies. Am J Epidemiol. 1976 Feb;103(2):152–165. doi: 10.1093/oxfordjournals.aje.a112213. [DOI] [PubMed] [Google Scholar]
- Lobmann M., Delem A., Jovanović D. Properties of A/Victoria/3/75 recombinants: development of an attenuated strain RIT 4050. Dev Biol Stand. 1977 Jun 1;39:43–46. [PubMed] [Google Scholar]
- Mackenzie J. S., Mackenzie I., Lloyd J., Dent V. Comparative trials of live attenuated and detergent split influenza virus vaccines. J Hyg (Lond) 1975 Dec;75(3):425–443. doi: 10.1017/s0022172400024499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. C., Zuckerman A. J., Beare A. S., Tyrrell D. A. Trials of Live Influenza Vaccine in the Royal Air Force. Br Med J. 1962 Apr 14;1(5284):1036–1042. doi: 10.1136/bmj.1.5284.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor T. E., Dick E. C., Dick C. R., Inhorn S. L. Attenuated influenza A vaccine (Alice) in an adult population: vaccine-related illness, serum and nasal antibody production, and intrafamily transmission. J Clin Microbiol. 1975 Nov;2(5):403–409. doi: 10.1128/jcm.2.5.403-409.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Chanock R. M. Temperature-sensitive mutants of influenza virus. II. Attenuation of ts recombinants for man. J Infect Dis. 1972 Aug;126(2):170–178. doi: 10.1093/infdis/126.2.170. [DOI] [PubMed] [Google Scholar]
- Prévost J. M., Peetermans J., Lamy F., Huygelen C. Immune response to vaccination with a live influenza virus (H3N2) vaccine ("Ann" strain). Infect Immun. 1973 Sep;8(3):420–424. doi: 10.1128/iai.8.3.420-424.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi G., Carlizza L., Andreoni M., Ragona G., Piga C., Pelosio A., Volpi A., Muzzi A. Protection from natural infection after live influenza virus immunization in an open population. J Hyg (Lond) 1979 Apr;82(2):231–236. doi: 10.1017/s002217240002564x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytel M. W., Jackson L. J., Ferstenfeld J. E., Rosenkranz M. A. New live attenuated influenza A/England/42/72 (H3N2) vaccine (Alice): reactogenicity,, immunogenicity, and protection efficacy. J Infect Dis. 1975 Dec;132(6):652–659. doi: 10.1093/infdis/132.6.652. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Steffenhagen K. A., Easterday B. C., Galasso G. J. Evaluation of 6-azauridine and 5-iododeoxyuridine in the treatment of experimental viral infections. J Infect Dis. 1976 Jun;133(6):603–612. doi: 10.1093/infdis/133.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]


