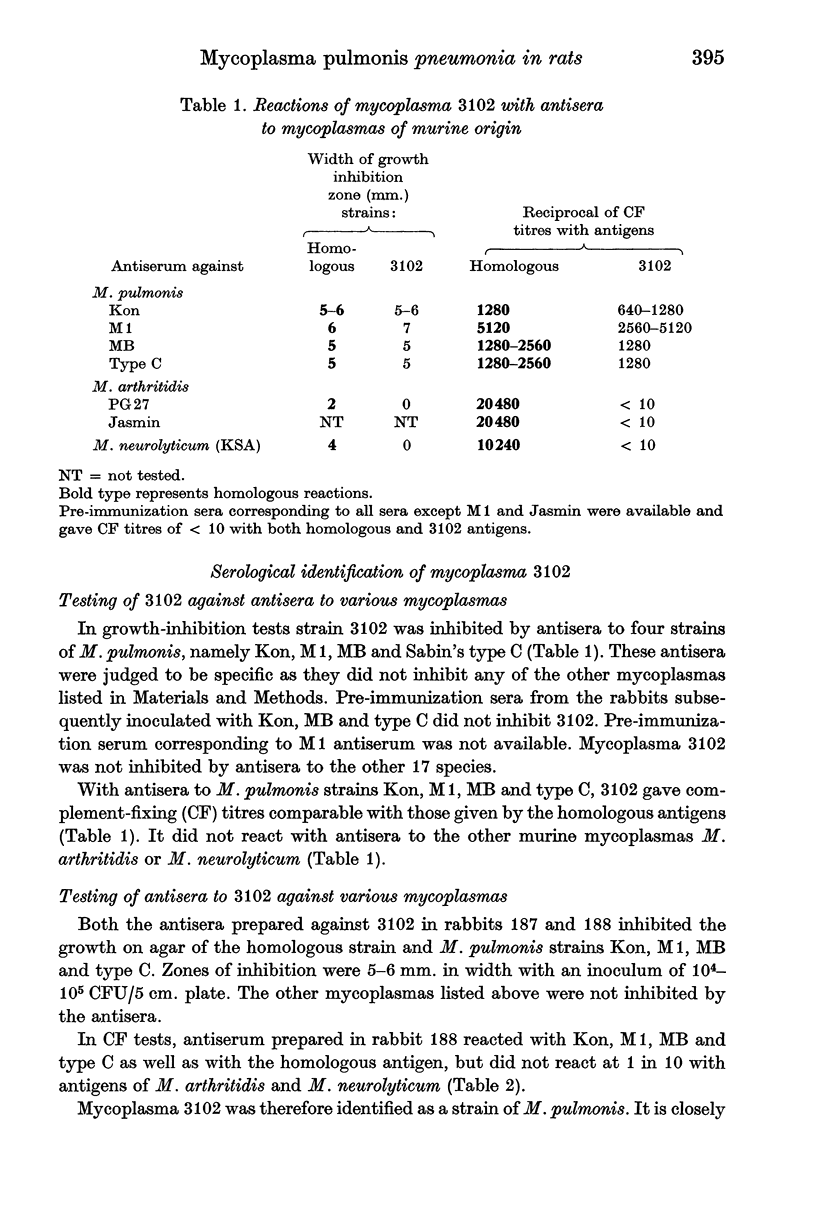
Abstract
Mycoplasma pulmonis was isolated from the pneumonic lung of a rat. Two groups of mycoplasma-free rats were inoculated, one with a culture of the M. pulmonis strain which had been cloned four times (group A) and the other with a lung homogenate of the rat from which the strain had been isolated (group B). A third group (C) consisted of uninoculated control animals. Each group was kept in strict isolation and allowed to breed so that the progeny was naturally exposed to any pathogens present in the inoculated animals. After different periods of exposure, rats were autopsied, respiratory tracts and inner ears were cultured for mycoplasmas and bacteria, and sera were tested for complement-fixing antibodies to murine mycoplasmas.
In group-A rats, M. pulmonis was consistently isolated from the inner ears or lungs from 50 to 715 days after exposure. Complement-fixing antibody to M. pulmonis was detected 20 days after inoculation, but in the naturally exposed progeny antibody took longer than 50 days to develop. Antibodies to the other known mycoplasmas of murine origin, M. arthritidis and M. neurolyticum, were never found. Purulent otitis interna was consistently found from day 55 onwards, while lung lesions were first observed at 85 days and persisted to 715 days. Pulmonary lesions developed more slowly in inoculated parents than in exposed progeny. Similar results were found in group-B rats, which were examined up to 441 days after inoculation. Uninoculated group-C rats were examined up to 768 days of age, but M. pulmonis was not recovered; of the 54 animals whose serum was tested all were negative to the three species of mycoplasmas, except one which had a titre of 16 with M. pulmonis. Pneumonia, bronchiectasis or lymphoreticular hyperplasia were not seen in any of these control rats. Bacterial respiratory pathogens were not isolated from rats in any of the groups, nor was antibody to Sendai virus detected.
The results suggest that M. pulmonis alone can cause pneumonia and bronchiectasis in rats since mechanical carry-over of another pathogen with the initial cloned inoculum is very unlikely and there was no evidence for the participation of any other rat pathogen. The respiratory disease induced by the cloned culture was comparable with that induced by the lung homogenate, and with the well-known syndrome of chronic respiratory disease and bronchiectasis in the rat.
Full text
PDF

















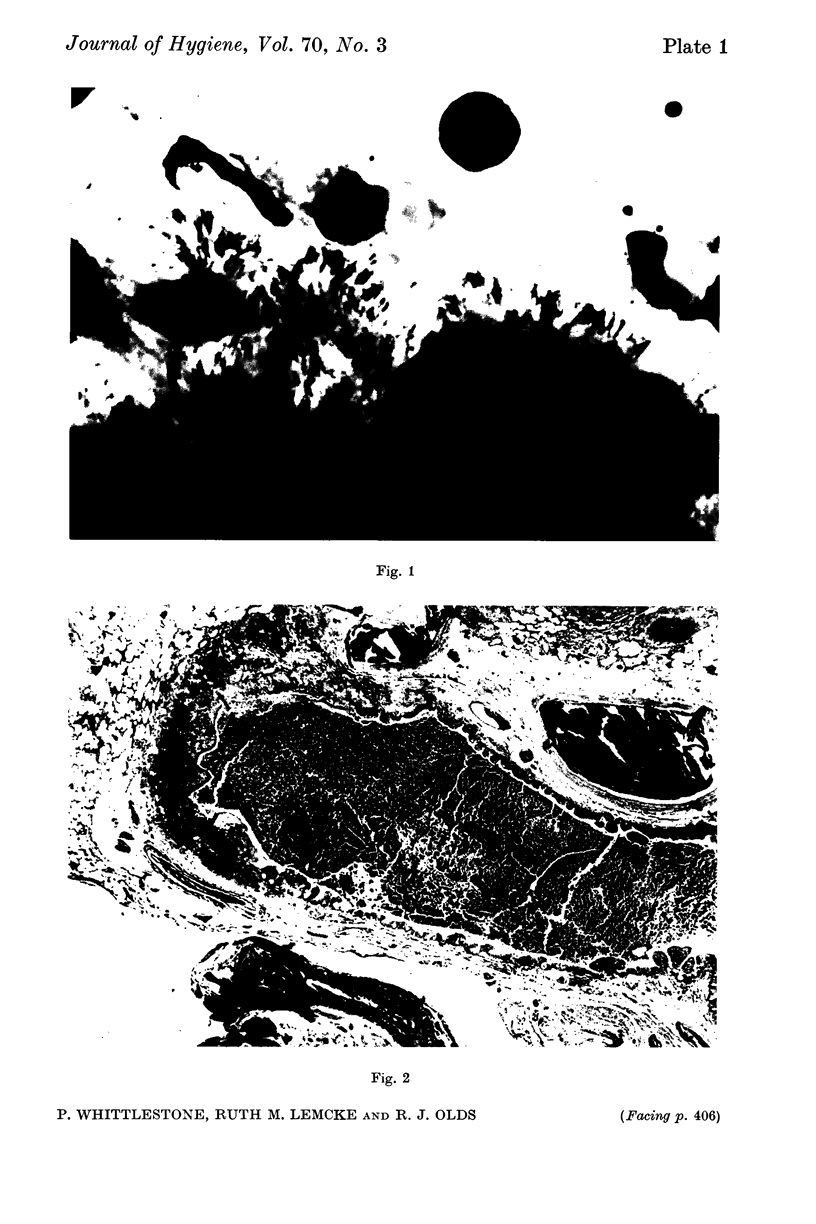


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAVER D. L., ASHBURN L. L., MCDANIEL E. G., BROWN N. D. LIPID DEPOSITS IN THE LUNGS OF GERM-FREE ANIMALS. Arch Pathol. 1963 Nov;76:565–570. [PubMed] [Google Scholar]
- Bell D. P., Elmes P. C. Effects of certain organisms associated with chronic respiratory disease on SPF and conventional rats. J Med Microbiol. 1969 Nov 4;2(4):511–519. doi: 10.1099/00222615-2-4-511. [DOI] [PubMed] [Google Scholar]
- Brennan P. C., Feinstein R. N. Relationship of hydrogen peroxide production by Mycoplasma pulmonis to virulence for catalase-deficient mice. J Bacteriol. 1969 Jun;98(3):1036–1040. doi: 10.1128/jb.98.3.1036-1040.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. C., Fritz T. E., Flynn R. J. Murine pneumonia: a review of the etiologic agents. Lab Anim Care. 1969 Jun;19(3):360–371. [PubMed] [Google Scholar]
- CLYDE W. A., Jr MYCOPLASMA SPECIES IDENTIFICATION BASED UPON GROWTH INHIBITION BY SPECIFIC ANTISERA. J Immunol. 1964 Jun;92:958–965. [PubMed] [Google Scholar]
- COUCH R. B., CATE T. R., CHANOCK R. M. INFECTION WITH ARTIFICIALLY PROPAGATED EATON AGENT (MYCOPLASMA PNEUMONIAE). IMPLICATIONS FOR DEVELOPMENT OF ATTENUATED VACCINE FOR COLD AGGLUTININ-POSITIVE PNEUMONIA. JAMA. 1964 Feb 8;187:442–447. [PubMed] [Google Scholar]
- Gay F. W. Association of a Mycoplasma-like agent with chronic pneumonia and bronchiectasis in the rat. J Bacteriol. 1969 Jan;97(1):441–444. doi: 10.1128/jb.97.1.441-444.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay F. W. Fine structure and location of the mycoplasma-like gray lung and rat pneumonia agents in infected mouse lung. J Bacteriol. 1967 Dec;94(6):2048–2061. doi: 10.1128/jb.94.6.2048-2061.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddens W. E., Jr, Whitehair C. K., Carter G. R. Morphologic and microbiologic features of trachea and lungs in germfree, defined-flora, conventional, and chronic respiratory disease-affected rats. Am J Vet Res. 1971 Jan;32(1):115–129. [PubMed] [Google Scholar]
- Goodwin R. F., Pomeroy A. P., Whittlestone P. Characterization of Mycoplasma suipneumonia: a mycoplasma causing enzootic pneumonia of pigs. J Hyg (Lond) 1967 Mar;65(1):85–96. doi: 10.1017/s0022172400045563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan P. C. Observations on the arthritogenic properties of Sabin's type C murine mycoplasma (Mycoplasma histotropicum). J Gen Microbiol. 1971 Aug;67(3):363–365. doi: 10.1099/00221287-67-3-363. [DOI] [PubMed] [Google Scholar]
- Hollingdale M. R., Lemcke R. M. Antigenic differences within the species Mycoplasma hominis. J Hyg (Lond) 1970 Sep;68(3):469–477. doi: 10.1017/s0022172400042376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingdale M. R., Lemcke R. M. The antigens of Mycoplasma hominis. J Hyg (Lond) 1969 Dec;67(4):585–602. doi: 10.1017/s0022172400042042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi N. N., Blackwood A. C., Dale D. G. Chronic Murine Pneumonia: A Review. Can J Comp Med Vet Sci. 1961 Nov;25(11):267–273. [PMC free article] [PubMed] [Google Scholar]
- Joshi N., Dale D. G., Blackwood A. C. Etiology of murine endemic pneumonia. Rev Can Biol. 1965 Sep;24(3):169–178. [PubMed] [Google Scholar]
- KLIENEBERGER-NOBEL E., CHENG K. K. On the association of the pleuro-pneumonia-like L3 organism with experimentally produced bronchiectasis in rats. J Pathol Bacteriol. 1955 Jul;70(1):245–246. doi: 10.1002/path.1700700125. [DOI] [PubMed] [Google Scholar]
- Kohn D. F., Kirk B. E. Pathogenicity of Mycoplasma pulmonis in laboratory rats. Lab Anim Care. 1969 Jun;19(3):321–330. [PubMed] [Google Scholar]
- LEMCKE R. M. A SEROLOGICAL COMPARISON OF VARIOUS SPECIES OF MYCOPLASMA BY AN AGAR GEL DOUBLE-DIFFUSION TECHNIQUE. J Gen Microbiol. 1965 Jan;38:91–100. doi: 10.1099/00221287-38-1-91. [DOI] [PubMed] [Google Scholar]
- LEMCKE R. M. THE SEROLOGICAL DIFFERENTIATION OF MYCOPLASMA STRAINS (PLEURO-PNEUMONIA-LIKE ORGANISMS) FROM VARIOUS SOURCES. J Hyg (Lond) 1964 Jun;62:199–219. doi: 10.1017/s0022172400039930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane-Petter W., Olds R. J., Hacking M. R., Lane-Petter M. E. Respiratory disease in a colony of rats. I. The natural disease. J Hyg (Lond) 1970 Dec;68(4):655–662. doi: 10.1017/s0022172400042595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemcke R. M., Forshaw K. A., Fallon R. J. The serological identity of Sabin's murine type C mycoplasma and Mycoplasma pulmonis. J Gen Microbiol. 1969 Sep;58(1):95–98. doi: 10.1099/00221287-58-1-95. [DOI] [PubMed] [Google Scholar]
- Lindsey J. R., Baker H. J., Overcash R. G., Cassell G. H., Hunt C. E. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971 Sep;64(3):675–708. [PMC free article] [PubMed] [Google Scholar]
- PANKEVICIUS J. A., WILSON C. E., FARBER J. F. The debatable role of a pleuropneumonialike organism in the etiology of chronic pneumonia in rats. Cornell Vet. 1957 Apr;47(2):317–325. [PubMed] [Google Scholar]
- Parker J. C., Cross S. S., Rowe W. P. Rat coronavirus (RCV): a prevalent, naturally occurring pneumotropic virus of rats. Arch Gesamte Virusforsch. 1970;31(3):293–302. doi: 10.1007/BF01253764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. C., Reynolds R. K. Natural history of Sendai virus infection in mice. Am J Epidemiol. 1968 Jul;88(1):112–125. doi: 10.1093/oxfordjournals.aje.a120859. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. A., Coid C. R. Sendai virus infection of rats as a convenient model of acute respiratory infection. Vet Rec. 1970 Feb 7;86(6):164–165. doi: 10.1136/vr.86.6.164. [DOI] [PubMed] [Google Scholar]
- Whittlestone P. Mycoplasma in enzootic pneumonia of pigs. Ann N Y Acad Sci. 1967 Jul 28;143(1):271–280. doi: 10.1111/j.1749-6632.1967.tb27666.x. [DOI] [PubMed] [Google Scholar]