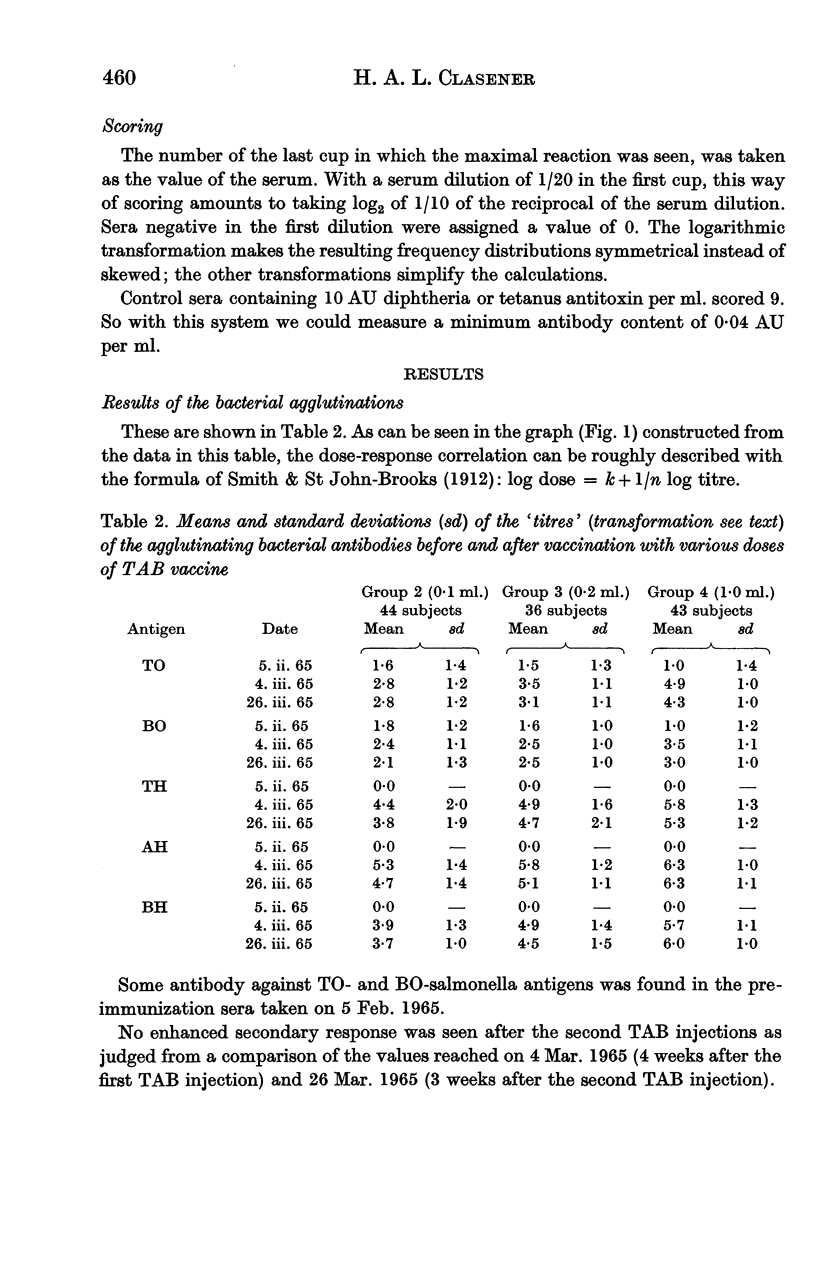
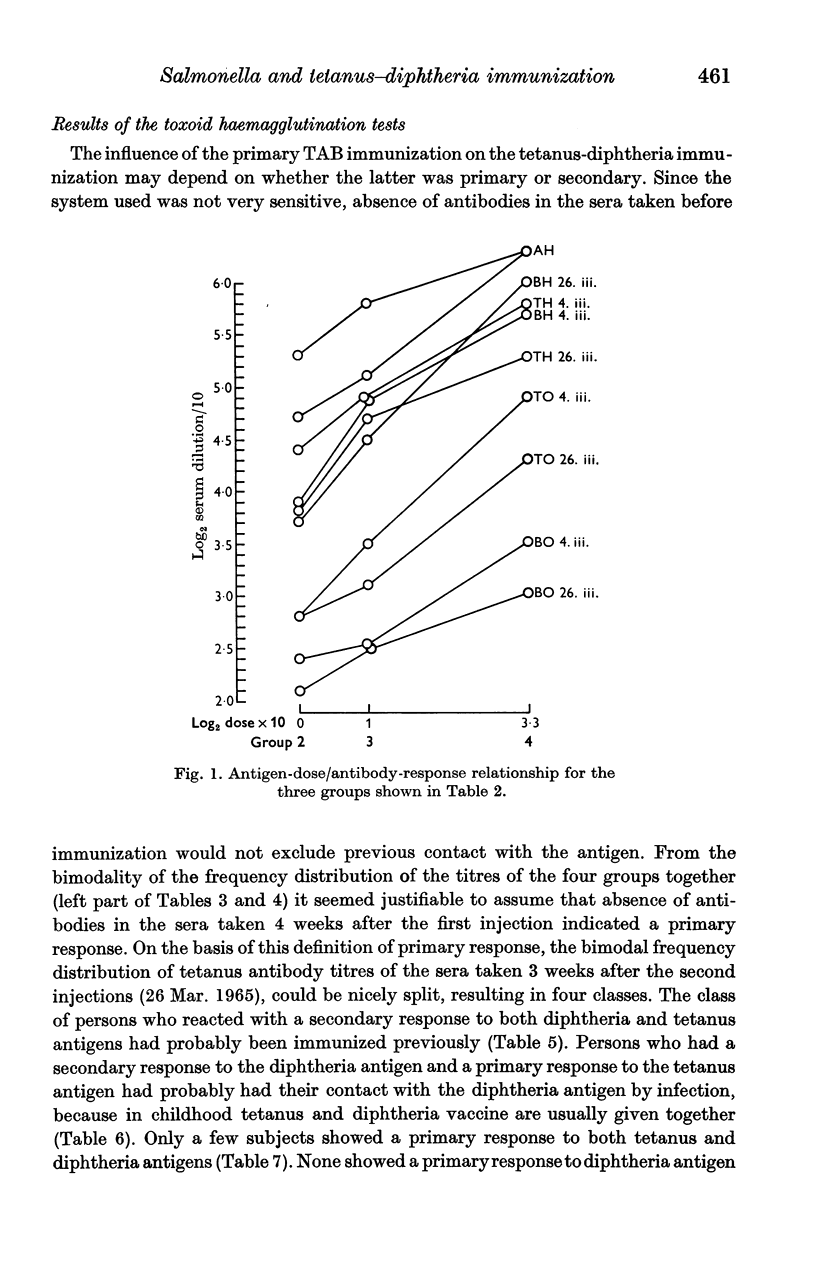
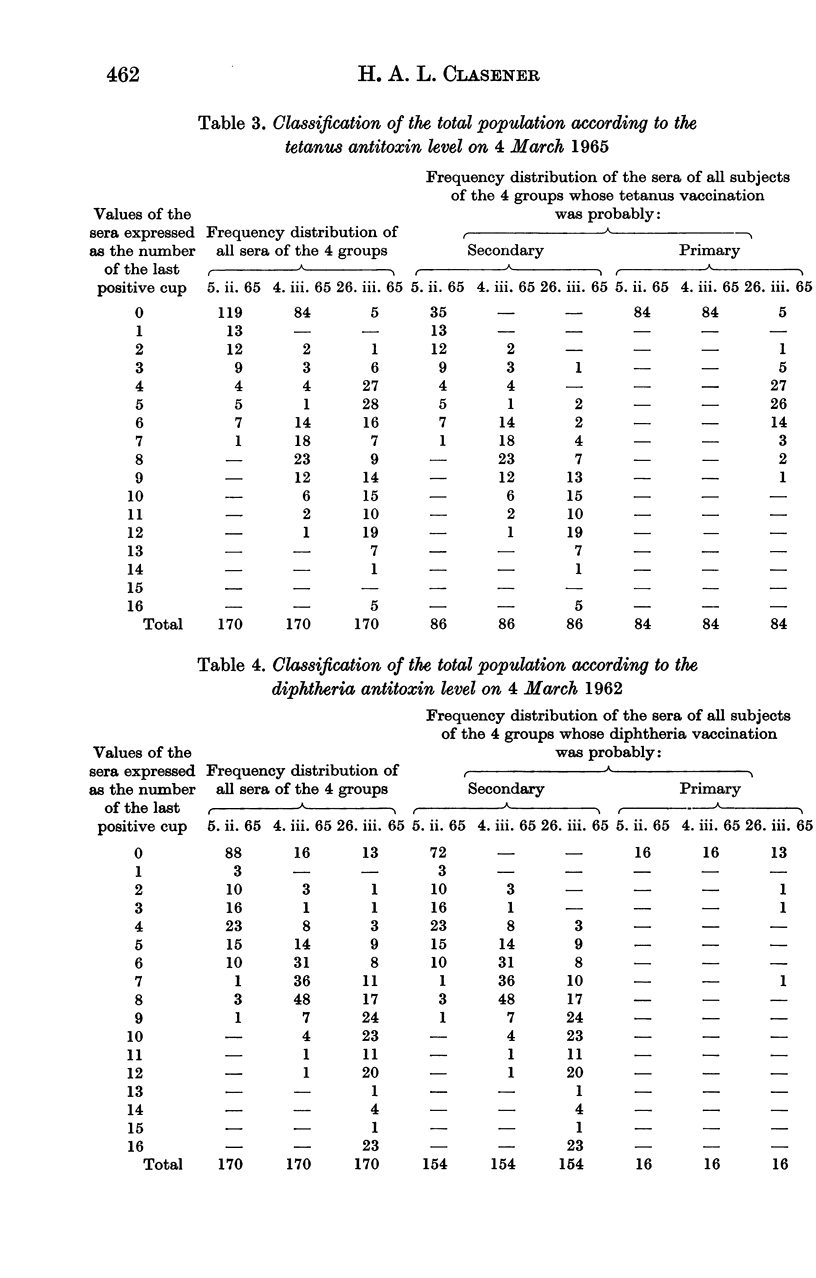
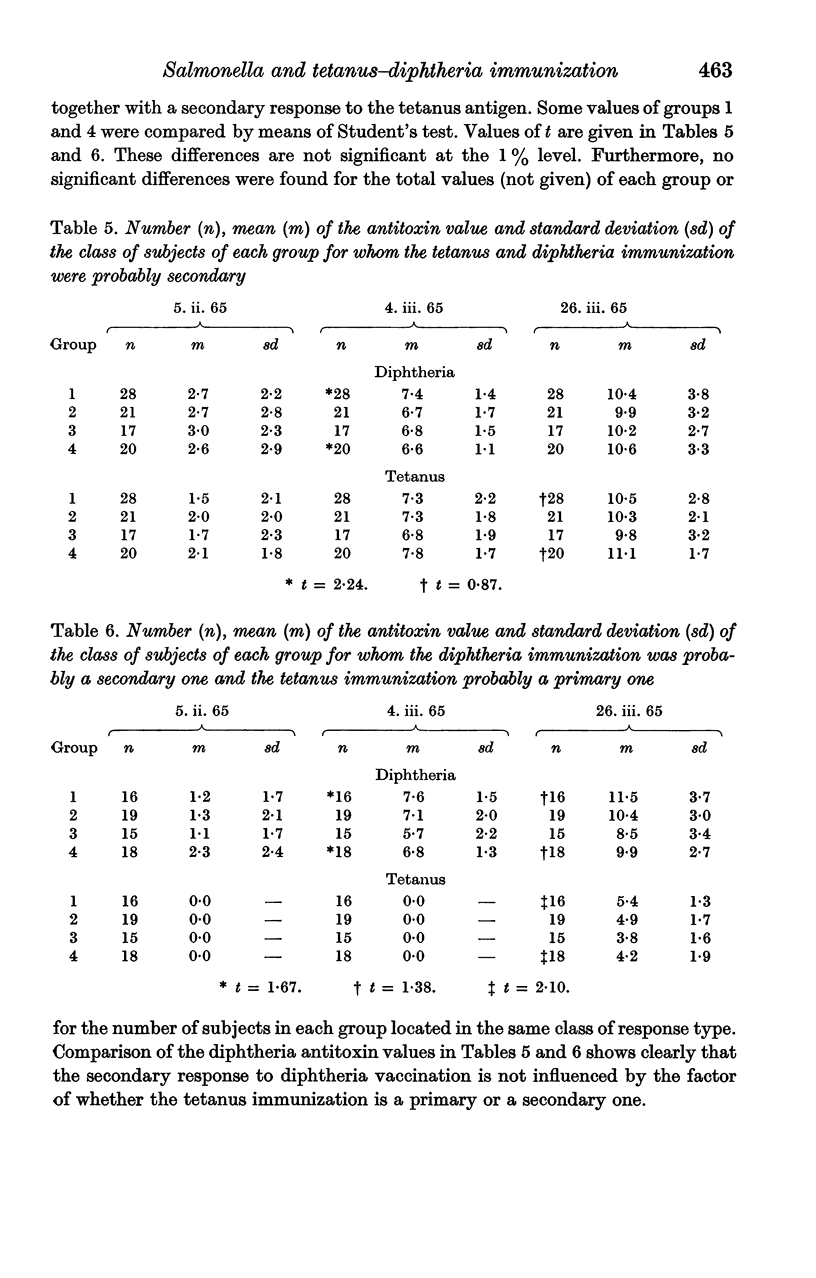
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHCROFT M. T., RITCHIE J. M., NICHOLSON C. C. CONTROLLED FIELD TRIAL IN BRITISH GUIANA SCHOOL CHILDREN OF HEAT-KILLED-PHENOLIZED AND ACETONE-KILLED LYOPHILIZED TYPHOID VACCINES. Am J Hyg. 1964 Mar;79:196–206. doi: 10.1093/oxfordjournals.aje.a120376. [DOI] [PubMed] [Google Scholar]
- BARR M., LLEWELLYN-JONES M. Some factors influencing the response of animals to immunisation with combined prophylactics. Br J Exp Pathol. 1953 Feb;34(1):12–22. [PMC free article] [PubMed] [Google Scholar]
- BAUER D. C., MATHIES M. J., STAVITSKY A. B. Sequences of synthesis of gamma-1 macroglobulin and gamma-2 globulin antibodies during primary and secondary responses to proteins, salmonella antigens, and phage. J Exp Med. 1963 Jun 1;117:889–907. doi: 10.1084/jem.117.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasener H. A. Immunization of man with typhoid and cholera vaccine. Agglutinating antibodies after intracutaneous and subcutaneous injection. J Hyg (Lond) 1967 Dec;65(4):449–456. doi: 10.1017/s0022172400045988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FECSIK A. I., BUTLER W. T., COONS A. H. STUDIES ON ANTIBODY PRODUCTION. XI. VARIATION IN THE SECONDARY RESPONSE AS A FUNCTION OF THE LENGTH OF THE INTERVAL BETWEEN TWO ANTIGENIC STIMULI. J Exp Med. 1964 Dec 1;120:1041–1049. doi: 10.1084/jem.120.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIBEL I. A comparative study on intracutaneous and haemagglutination procedures for assaying diphtheria antitoxin, with special reference to the avidity of the antitoxin. Acta Pathol Microbiol Scand. 1956;39(6):455–468. doi: 10.1111/j.1699-0463.1956.tb05073.x. [DOI] [PubMed] [Google Scholar]
- STAVITSKY A. B. Micromethods for the study of proteins and antibodies. I. Procedure and general applications of hemagglutination and hemagglutination-inhibition reactions with tannic acid and protein-treated red blood cells. J Immunol. 1954 May;72(5):360–367. [PubMed] [Google Scholar]
- STEVENS K. M. Some considerations of the antigen dose-antibody response relationship. J Immunol. 1956 Mar;76(3):187–191. [PubMed] [Google Scholar]
- SURJAN M., NYERGES G. Diphtheria antitoxin titration of human sera by haemagglutination. Z Immun exp ther. 1962 Dec;124:401–410. [PubMed] [Google Scholar]
- SURJAN M., NYERGES G. Haemagglutination procedure for the assay of tetanus antitoxin of children's sera. Z Immun exp ther. 1962 Dec;124:390–400. [PubMed] [Google Scholar]
- TASMAN A., van RAMSHORST J., SMITH L. Determination of diphtheria and tetanus antitoxin with the aid of haemagglutination. Antonie Van Leeuwenhoek. 1960;26:413–429. doi: 10.1007/BF02539029. [DOI] [PubMed] [Google Scholar]
- UHR J. W., FINKELSTEIN M. S. Antibody formation. IV. Formation of rapidly and slowly sedimenting antibodies and immunological memory to bacteriophage phi-X 174. J Exp Med. 1963 Mar 1;117:457–477. doi: 10.1084/jem.117.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]


