Abstract
We have previously shown that glutathione (GSH) and glutathione disulfide interact with metallothionein (MT) and modulate its capacity to donate and transfer zinc. In this paper, we show that ATP also forms a 1:1 complex with MT (Kd = 176 ± 33 μM, pH 7.4) that enhances the transfer of zinc to zinc-depleted sorbitol dehydrogenase, increases the rate of thiol–disulfide interchange with Ellman’s reagent [5,5′-dithiobis (Z-nitrobenzoic acid)], and changes the apparent shape of the protein. GTP produces almost identical effects. The corresponding di- or monophosphates and pyrimidine nucleotides, however, neither bind as strongly as ATP nor enhance zinc transfer. Carbamoylation of MT lysines abolishes ATP binding, indicating that these highly conserved residues are part of the binding site. GSH decreases, whereas glutathione disulfide increases, ATP binding. The interaction of MT with two critical cellular ligands, i.e., GSH and ATP, and ensuing effects on zinc transfer and reactivity suggest that MT is not merely a cellular zinc buffer but, rather, actively participates in zinc distribution. Apparently, when isolated, MT lacks two important effectors that affect its redox behavior and function. The magnitude of the binding constant and the cellular concentration of ATP indicate that in the cell MT could be essentially saturated with ATP at low concentrations of GSH. Both the redox and energy states of the cell seem to control zinc distribution from MT, but their relative contributions require further studies.
Seven zinc atoms bound to 20 cysteine sulfur atoms form the two metal clusters of metallothionein (MT) and constitute a reservoir for storage and distribution of zinc (1). The cluster structure forms a network of zinc–sulfur interactions that have no precedent in the nonliving world. The protein envelops the zinc atoms, each of which is bound to four thiolate ligands, in a manner that effectively shields them from the environment. Ligand exchange and redox reactions of the cysteine sulfur donor atoms are responsible for the kinetic lability of zinc in the thermodynamically stable sites of MT (2). The cluster unit works by a mechanism that allows the cysteine sulfur ligands to zinc to be oxidized, allowing an oxidoreductive mechanism to modulate affinity of the otherwise inert zinc atom (3). We have shown further that the interactions of MT with glutathione (GSH) and glutathione disulfide (GSSG) (4) or other oxidizing agents (5) control the state of zinc in MT in vitro, suggesting that the zinc content of MT is a function of the cellular redox state. Aside from GSH (6), the only other biological ligand known to bind to MT, until now, is phosphate that appears to be required for the formation of stable MT crystals (7). It was found to be localized between the two protein domains bound to the carbonyl group of Cys-19 and to the ɛ-amino group of Lys-31, which forms the only interdomain hydrogen bonds with the carbonyl groups of Cys-19 and Cys-21 (7). It has also been found to bind to dimeric Cd-MT with relatively high affinity (14 μM at pH 7.4) (8) or to monomeric Cd-MT in the presence of excess cadmium (9). The physiological significance of phosphate binding, if any, is not known.
Because it was reported earlier that GTP binds to MT (10), we extended our search for nucleotides as ligands of MT and found that both ATP and GTP bind to MT. This interaction, seemingly limited to purine nucleotides, may be both structurally and functionally significant in the light of our recent studies of the MT system that addressed the coordination dynamics of zinc, i.e., its transfer to and from MT, and the reactivity of the cysteine ligands in the clusters (3).
MATERIALS AND METHODS
Materials.
Nucleotides, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), rabbit liver Cd,Zn-metallothionein II (Cd,Zn-MT-2), and protein molecular weight markers were obtained from Sigma; 4-(2-pyridylazo)resorcinol was from Aldrich; 65ZnCl2 (77.7–103.6 Gbq/g) was from Dupont/NEN; sheep liver sorbitol dehydrogenase (SDH) was from Boehringer Mannheim; and adenosine 5′-[β,γ-imido]triphosphate (AMP-PNP) was from Fluka. Zinc-depleted sorbitol dehydrogenase (apo-SDH) and human Zn7-MT-2 were prepared and characterized as described (4).
Zinc Transfer from MT to apo-SDH in the Presence of Nucleotides.
Apo-SDH (1.7 μM) was incubated with Zn7-MT (0.24 μM) and the nucleotide (1 mM) to be tested in 0.2 M Tris⋅HCl (pH 7.4) at 22.5°C. Aliquots were withdrawn periodically and assayed for enzymatic activity (4).
Reaction of 65Zn-MT-2 with ATP.
Human 65Zn7-MT-2 (4) (0.76 μM) was incubated with 1 mM ATP at 22.5°C for 1 h. The reaction mixture was then separated on a DEAE MemSep-1000 chromatography cartridge (Millipore) using a linear 10-min gradient from 0 to 75 mM NaCl in 10 mM Tris⋅HCl (pH 8.6) at a flow rate of 5 ml/min. Radioactivity in each fraction was measured by γ-emission spectroscopy with a Searle model 1185 Automatic Gamma system operating at a 0.12–1.2 MeV energy range (1 eV = 1.602 × 10–9 J).
Gel Filtration Experiments.
To study ATP binding (11), the Sephadex G-25 column (1 × 30 cm) was equilibrated with 50 mM Hepes⋅NaOH (pH 7.4) containing various amounts of ATP (50, 100, 200, and 600 μM) at 25°C. Cd,Zn-MT-2 was dissolved in this buffer and then applied to the column at a flow rate of 6 ml/h. If ATP binds to MT, the resulting elution profile monitored at 259 nm will exhibit a peak at the position where MT elutes, and a trough, representing the depletion of ATP in the buffer, at the position of ATP elution. From the area of the trough and the known amount of protein applied, the binding ratio was determined at each concentration of ATP.
To assess possible changes in the shape of MT, a Sephadex G-75 column (1 × 100 cm) was equilibrated with the same Hepes buffer but containing 10 mM NaCl and operated at a flow-rate of 6 ml/h. For this column, a linear calibration of the elution volumes against the logarithm of the molecular weights was obtained with the following proteins: oxidized A chain of bovine insulin, bovine trypsin inhibitor, horse cytochrome c, bovine carbonic anhydrase, and ovalbumin (Pharmacia). MT was analyzed spectrophotometrically at 343 nm by titration of sulfhydryls with 2,2′-dithiodipyridine.
Lysine Modification.
Lysines of MT were modified by dissolving rabbit liver Cd,Zn-MT-2 in 0.2 ml of saturated sodium borate (pH 9.2), adding solid potassium cyanate to a final concentration of 1 M, and incubating the reaction mixture for 40 h at 37°C (12). Excess salt was removed by at least six dilution/concentration cycles using Centricon 3 centrifugal microconcentrators (Amicon).
Reactivity of MT in the Presence of Nucleotides.
The thiol reactivity of MT was determined by using Ellman’s reagent [5,5′-dithiobis(2-nitrobenzoic acid)] under pseudo-first-order rate conditions where [DTNB] ≪ [MT thiols] (13). Under these conditions two equivalents of thionitrobenzoate are formed, because the inter- or intramolecular disulfide of MT is favored over the mixed disulfide (14). The reaction between MT (10 μM) and DTNB (4 μM) without or with nucleotide (1 mM) in 0.2 M Tris⋅HCl (pH 7.4) was followed spectrophotometrically at 412 nm and 25°C.
RESULTS
Zinc Transfer from MT to apo-SDH in the Presence of Nucleotides.
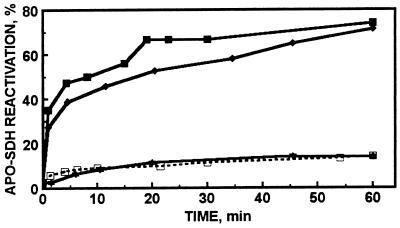
In the absence of any other agents the reconstitution of apo-SDH with Zn7-MT-2 is slow but reaches 13% after 1 h of incubation at equimolar concentrations of apo-SDH and zinc in MT (k2 = 16 M−1⋅s−1; Fig. 1). Full activation requires equimolar concentrations of apo-SDH and MT with regard to protein, and under these conditions only one of the seven zinc atoms of MT is transferred (4). If ATP (1 mM) is present with equimolar concentrations of apo-SDH and zinc in MT the rate of the reaction increases about 18-fold (k2 = 288 M−1⋅s−1) and its extent increases almost 6-fold, leading to 74% reactivation (Fig. 1). This comparison of rate constants is based on the assumption that only one zinc atom is transferred in both cases. However, in the presence of ATP, MT transfers at least five zinc atoms to apo-SDH. The enhancement of zinc transfer by ATP is concentration dependent; 10 μM ATP leads to 21% and 100 μM leads to 36% reactivation after 1 h, respectively (data not shown). ATP (1 mM) does not affect the enzymatic activity of SDH. GTP (1 mM) produces similar increases in the rate of zinc transfer from MT, 13-fold (k2 = 207 M−1⋅s−1), and in the number of zinc atoms transferred, four atoms. These effects are of the same order of magnitude as those observed with the GSH–GSSG system (4). Other purine nucleotides [e.g., ADP (Fig. 1), AMP, GMP, cAMP, and cGMP], pyrimidine nucleotides [such as UTP or CTP], phosphate, or pyrophosphate do not enhance zinc transfer from MT at all. Neither calcium nor magnesium ions at concentrations of up to 1 mM affect this reaction in any way.
Figure 1.
Zinc transfer from human Zn7-MT to apo-SDH in the presence of ATP (■), AMP-PNP (⧫), and ADP (★), and in the absence of nucleotides (□). Apo-SDH (1.7 μM) was incubated with 0.24 μM MT in 0.2 M Tris⋅HCl (pH 7.4) at 22.5°C in the absence or presence of nucleotide (1 mM). Aliquots were withdrawn periodically and assayed for enzymatic activity.
ATP analogs such as adenosine 5′-[γ-thio]triphosphate and AMP-PNP are hydrolyzed poorly and, therefore, are commonly used to examine whether or not ATP hydrolysis is a component or accompaniment of a physiological process. The reactivation of apo-SDH by MT in the presence of AMP-PNP closely resembles that in the presence of ATP, indicating that ATP hydrolysis is not essential for zinc transfer from MT (Fig. 1). Moreover, vanadate, a potent ATPase inhibitor, does not affect the enhancement of zinc transfer by ATP.
ATP is a relatively weak zinc-chelating agent [log K = 5.2 (15)] with a binding constant for zinc close to that of zincon [log K = 4.9 (16)]. To examine whether or not the effect of ATP solely relates to its capacity to sequester zinc, we have studied the effect of zincon on zinc transfer for comparison. Zincon fails to enhance zinc transfer from MT to apo-SDH. Three types of experiments support the conclusion that of itself ATP does not release zinc from MT. (i) When 4-(2-pyridylazo)resorcinol is used as the zinc acceptor, ATP (1 mM) does not induce zinc transfer from MT. (ii) ATP also does not displace zinc from 65Zn-labeled MT, as demonstrated by anion-exchange chromatography. (iii) Determination of zinc by atomic absorption spectrophotometry in fractions obtained by Hummel–Dreyer chromatography in the presence of ATP (see below) show that at most 7% of zinc is released from MT.
Binding of ATP to MT.
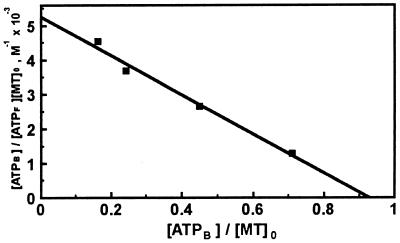
Experiments using the Hummel–Dreyer method (11) established the binding of ATP to MT. The linear Scatchard plot (R2 = 0.9854) gives a value of 0.93 for the number of identical and independent binding sites, indicating that one molecule of ATP binds to MT, with a Kd value of 176 ± 33 μM (Fig. 2). The elution profile for GTP is similar, but the elution profile for UTP does not indicate binding of this nucleotide, and the small changes observed with ADP are within the limits of experimental error. Thus, preferential binding seems to be limited to purine nucleotides, which are also the ones that affect zinc transfer from MT (see above).
Figure 2.
Scatchard plot of ATP binding to rabbit Cd,Zn-MT-2. Binding data were obtained by the Hummel–Dreyer method. Identical results were obtained when human Zn7-MT-2 was used.
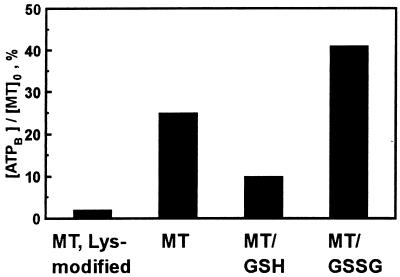
In mammalian MT-1 and -2 isoforms, eight lysine residues are highly conserved and might be loci for ATP binding. These residues had been modified previously by carbamoylation for different reasons (12). We therefore used this procedure and find that it essentially abolishes ATP binding (Fig. 3). Further, although zinc is transferred from lysine-modified MT to apo-SDH, this transfer is not enhanced by ATP, demonstrating that ATP binding is a prerequisite for the enhancement of zinc transfer. This conclusion is also supported by the close agreement between the binding data and the dependence of zinc transfer on the concentration of ATP (see above). Because phosphate is known to bind to Lys-31 in MT crystals (7), we investigated the binding of ATP in the presence of phosphate. ATP binding is not detected in the presence of a relatively large excess of phosphate (50 mM), suggesting that phosphate and ATP compete for a binding site that includes Lys-31.
Figure 3.
Effect of GSH and modification of lysines on ATP binding to MT. ATP (100 μM) was incubated with rabbit Cd,Zn-MT-2 (142 μM) in 50 mM Hepes⋅NaOH (pH 7.4) in the absence of GSH or in the presence of either GSH (120 μM) or GSSG (500 μM) (4). Binding was determined by the Hummel–Dreyer method.
Binding of ATP to MT in the Presence of GSH.
We have proposed that the cellular GSH redox state controls the transfer of zinc from MT (4, 17). Hence, the effect of GSH and GSSG on the binding of ATP to MT was also examined; the results clearly show that GSH inhibits ATP binding to MT but that GSSG enhances it (Fig. 3).
Sulfhydryl Reactivity and Conformational Changes of MT in the Presence of Nucleotides.
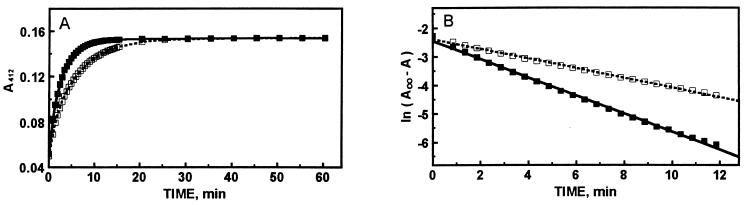
The reactivity of the sulfhydryl groups of MT is one of the most sensitive probes of its conformation (16). To determine whether or not nucleotide binding affects the reactivity of the molecule, we have investigated the reaction of MT with DTNB under pseudo-first-order rate conditions, i.e., [DTNB] ≪ [MT thiols]. ATP increases the rate of the reaction significantly (Fig. 4A). Kinetic analysis of the data demonstrates that the reaction can be described by a single process with a rate constant of 2.8 ± 0.3 × 10−3 s−1 (n = 2) in the absence and of 5.3 ± 0.4 × 10−3 s−1 (n = 2) in the presence of ATP (Fig. 4B). Similarly, GTP increases the reactivity 2.2-fold; AMP has no effect, however.
Figure 4.
Reactivity of thiols of MT in the absence and presence of ATP. (A) Human MT-2 (10 μM) was incubated with (■) or without (□) ATP (1 mM) and DTNB (4 μM) in 0.2 M Tris⋅HCl (pH 7.4) and the reaction was followed spectrophotometrically. (B) First-order replot of the data given in A.
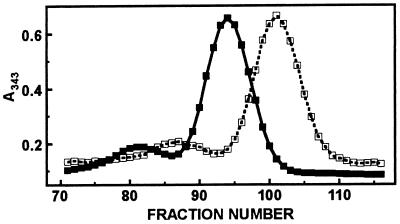
The shape of the MT molecule is that of a prolate ellipsoid (dumbbell) and thus deviates significantly from that of a globular protein. Hence, there is a large difference between the apparent molecular weight determined by gel filtration and the true molecular weight of 6,000 of MT (18). On a calibrated gel filtration column, the MT–ATP complex was eluted significantly later than MT (Fig. 5), demonstrating a large change in the shape of MT upon ATP binding. The different elution volumes correspond to apparent molecular weights of 16,500 in the absence of ATP and of 11,200 in its presence. GTP has a similar effect, but AMP does not affect the elution behavior of MT.
Figure 5.
Gel filtration of MT and of the MT–ATP complex. Rabbit Cd,Zn-MT-2 (140 μM) was subjected to gel filtration in the absence (■) and presence (□) of 1 mM ATP. For the latter experiment the Sephadex G-75 column was equilibrated in the same buffer (50 mM Hepes⋅NaOH, pH 7.4/10 mM NaCl), but containing 1 mM ATP. Fractions of 0.6 ml were collected.
DISCUSSION
MT does not have a consensus ATP or GTP binding motif (19). In contrast to other systems with ATP/GTP recognition sites, where ATP binds in the micromolar and GTP in the nanomolar range, the binding of ATP to MT is relatively weak, ruling out conventional assays for tight binding. For this reason, we used an equilibrium method proposed by Hummel and Dreyer (11) to determine the stability constant of the MT–ATP complex. Though weak, the binding seems to be specific for purine nucleotide triphosphates, because binding of pyrimidine nucleotides could not be detected. The binding data for nucleotides correlate well with corresponding data for zinc transfer from MT to apo-SDH, indicating that binding of the nucleotide is required for the enhancement of zinc transfer. It is significant that these effects were seen only for the purine nucleotides. They are known to result in more stable zinc complexes because of the formation of a macrochelate involving N-7 of the nucleobase (15). In two cases at least, interaction of N-7 of ATP with a zinc atom of an enzyme has been demonstrated (20, 21). Purine nucleotides might also act as shuttle agents for zinc (5) and this would demand that the nucleotide not be bound very strongly. Although Fe–ATP complexes have been isolated from the cytosolic low molecular weight iron pool (22), there does not seem to be any record of any biochemical study indicating how much cellular zinc is bound to either ATP or GTP. However, it has been observed that MT activates pyridoxal kinase, which requires a Zn–ATP complex (23).
The concentration of cellular ATP is 3–5 mM (see below), approximately one order of magnitude more than that of GTP, which is estimated to be 0.3–0.6 mM (24). Because MT would be expected to form complexes with ATP preferentially, we are herein restricting our discussion to this nucleotide. We are presently investigating the possible roles of high energy phosphates in the MT system, in particular, because a relation of MT and/or zinc in the regulation of energy balance has now been implied on the basis of the observation that mice with targeted disruption of MT-1 and -2 genes become obese (25).
Experiments with lysine-modified MT show that these residues are involved in ATP binding (Fig. 3), among them certainly Lys-31, as this residue binds phosphate in the crystal structure of MT, which was, indeed, found essential to the crystallization and subsequent structure determination by x-ray diffraction (7). The eight lysines conserved in the protein have been postulated to be necessary to stabilize the negatively charged clusters (26). Lysine-modified MT still binds all seven zinc atoms (12). In addition, mutagenesis experiments, in which all lysines of the α-domain are replaced with glutamates gave no evidence that the lysines are critical for the structure of the protein (27) and their role has remained undefined. Our results suggest that the lysines are conserved because of their role in nucleotide binding though they could also be part of a nuclear localization sequence. Nuclear retention of MT requires energy and the “nucleophilic” distribution of MT in human tumor cells has been related to the ATP status (28).
Given the relatively low affinity of ATP for MT, the question arises whether or not in the cell MT is associated with ATP. Taking into account its tight control, the cytosolic concentration of ATP is almost always in the millimolar range (29, 30), whereas that of MT seems to be in the micromolar range or below (31). Judging from the sparse data available, one calculates that in the cells studied so far cytosolic MT would be essentially saturated with ATP. We have previously discussed that given the typical cellular concentrations of GSH, MT would also be saturated with GSH (4). GSH synthesis itself is ATP-dependent and GSH concentrations seem to vary over a much wider range than those of ATP, i.e., between 0.1 and 10 mM (32). Because GSH inhibits ATP binding (Fig. 3) and binds about one order of magnitude stronger than ATP (6), the amount of ATP bound to MT in the cell will depend on the concentration of GSH. These data reinforce our conclusion that the concentration of GSH is an important modulator of the transfer reactions of MT (4). Thus at least two ligands, ATP and GSH, seem to bind to MT in vivo, although when isolated, MT is always devoid of these “effectors.” In their absence MT adopts a conformation in which the zinc atoms are almost completely shielded from solvent (7, 33).
The observation that ATP enhances zinc transfer from MT to apo-SDH indicates that ATP changes the conformation of MT presumably to bring about more efficient zinc transfer. The small binding energy resulting from rather weak ATP binding might nevertheless induce a substantial conformational change in a rather flexible small protein such as MT. Indeed, the large change in the elution profile of the MT–ATP complex in comparison with MT alone in a gel filtration experiment corresponds to a difference of 5,300 in apparent molecular weights (Fig. 5) and suggests such a conformational change. The fact that the MT–ATP complex is eluted later than MT would seem to suggest further that the shape of the MT molecule changes from that of a dumbbell to that of a more globular protein.
Our experiments show that neither effector releases zinc of itself but each activates the protein for reactions with oxidants such as disulfides. Thus ATP modulates the redox behavior of MT in a manner analogous to that of GSH. GSH has been proposed to bind to the β-domain of MT by displacing a thiol ligand (6). We have suggested that this free thiol might be more reactive toward disulfides such as GSSG (4). Herein, we have used Ellman’s reagent to investigate the redox behavior of MT, a reaction that has been studied earlier in some detail (13, 14, 34). Under the conditions of our experiments, the kinetic phase affected by ATP corresponds to the faster of the two phases ascribed by others to the reaction of the α-domain of MT (35). If these assignments are correct, then GSH and ATP would act on separate domains and activate the zinc ligands for reactions that ultimately might be the two keys necessary for opening each cluster for zinc transfer.
Acknowledgments
This work was supported by the Endowment for Research in Human Biology, Inc.
ABBREVIATIONS
- MT
metallothionein
- MT-2
metallothionein isoform 2
- GSH
glutathione
- GSSG
glutathione disulfide
- SDH
sorbitol dehydrogenase
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid)
- AMP-PNP
adenosine 5′-[β,γ-imido]triphosphate
- Cd
Zn-MT-2, Cd,Zn-metallothionein II
References
- 1.Vallee B L, Maret W. In: Metallothionein III. Suzuki K T, Imura N, Kimura M, editors. Basel, Switzerland: Birkhäuser; 1993. pp. 1–27. [Google Scholar]
- 2.Maret W, Larsen K S, Vallee B L. Proc Natl Acad Sci USA. 1997;94:2233–2237. doi: 10.1073/pnas.94.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang L J, Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3483–3488. doi: 10.1073/pnas.95.7.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob C, Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3489–3494. doi: 10.1073/pnas.95.7.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouwer M, Brouwer T H, Cashon R E. Biochem J. 1993;294:219–225. doi: 10.1042/bj2940219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins A H, McRee D E, Williamson M, Collett S A, Xuong N H, Furey W F, Wang B C, Stout C D. J Mol Biol. 1991;221:1269–1293. [PubMed] [Google Scholar]
- 8.Palumaa P, Vašák M. Eur J Biochem. 1992;205:1131–1135. doi: 10.1111/j.1432-1033.1992.tb16882.x. [DOI] [PubMed] [Google Scholar]
- 9.Palumaa P, Zerbe O, Vašák M. Biochemistry. 1993;32:2874–2879. doi: 10.1021/bi00062a019. [DOI] [PubMed] [Google Scholar]
- 10.Vallee B L. Experientia Suppl. 1979;34:19–40. doi: 10.1007/978-3-0348-6493-0_1. [DOI] [PubMed] [Google Scholar]
- 11.Hummel J P, Dreyer W J. Biochim Biophys Acta. 1962;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- 12.Zeng J. Methods Enzymol. 1991;205:433–437. doi: 10.1016/0076-6879(91)05127-h. [DOI] [PubMed] [Google Scholar]
- 13.Cismowski M J, Huang P C. Biochemistry. 1991;30:6626–6632. doi: 10.1021/bi00240a036. [DOI] [PubMed] [Google Scholar]
- 14.Savas M M, Shaw C F, III, Petering D H. J Inorg Biochem. 1993;52:235–249. doi: 10.1016/0162-0134(93)80028-8. [DOI] [PubMed] [Google Scholar]
- 15.Sigel H, Song B. Metal Ions Biol Syst. 1996;32:135–205. [Google Scholar]
- 16.Shaw C F, III, Savas M M, Petering D H. Methods Enzymol. 1991;205:401–414. doi: 10.1016/0076-6879(91)05122-c. [DOI] [PubMed] [Google Scholar]
- 17.Maret W. Proc Natl Acad Sci USA. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kägi J H R, Himmelhoch S R, Whanger P D, Bethune J L, Vallee B L. J Biol Chem. 1974;249:3537–3542. [PubMed] [Google Scholar]
- 19.Kjeldgaard M, Nyborg J, Clark B F C. FASEB J. 1996;10:1347–1368. [PubMed] [Google Scholar]
- 20.Wu F Y-H, Huang W-J, Sinclair R B, Powers L. J Biol Chem. 1992;267:25560–25567. [PubMed] [Google Scholar]
- 21.Wilson D K, Quiocho F A. Biochemistry. 1993;32:1689–1694. doi: 10.1021/bi00058a001. [DOI] [PubMed] [Google Scholar]
- 22.Weaver J, Pollack S. Biochem J. 1989;261:787–792. doi: 10.1042/bj2610787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Churchich J E, Scholz G, Kwok F. Biochim Biophys Acta. 1989;996:181–186. doi: 10.1016/0167-4838(89)90245-8. [DOI] [PubMed] [Google Scholar]
- 24.Hatakeyama K, Harada T, Kagamiyama H. J Biol Chem. 1992;267:20734–20739. [PubMed] [Google Scholar]
- 25.Beattie J H, Wood A M, Newman A M, Bremner I, Choo K H A, Michalska A E, Duncan J S, Trayhurn P. Proc Natl Acad Sci USA. 1998;95:358–363. doi: 10.1073/pnas.95.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pande J, Vašák M, Kägi J H R. Biochemistry. 1985;24:6717–6722. doi: 10.1021/bi00344a062. [DOI] [PubMed] [Google Scholar]
- 27.Pan P K, Hou F Y, Cody C W, Huang P C. Biochem Biophys Res Commun. 1994;202:621–628. doi: 10.1006/bbrc.1994.1973. [DOI] [PubMed] [Google Scholar]
- 28.Woo E S, Kondo Y, Watkins S C, Hoyt D G, Lazo J S. Exp Cell Res. 1996;224:365–371. doi: 10.1006/excr.1996.0146. [DOI] [PubMed] [Google Scholar]
- 29.Peters G J, De Arbreu R A, Oosterhof A, Veerkamp J H. Biochim Biophys Acta. 1983;759:7–15. doi: 10.1016/0304-4165(83)90182-4. [DOI] [PubMed] [Google Scholar]
- 30.Rauch U, Schulze K, Witzenbichler B, Schultheiss H P. Circ Res. 1994;75:760–769. doi: 10.1161/01.res.75.4.760. [DOI] [PubMed] [Google Scholar]
- 31.Krezoski S K, Villalobos J, Shaw C F, III, Petering D H. Biochem J. 1998;255:483–491. [PMC free article] [PubMed] [Google Scholar]
- 32.Meister A. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 33.Arseniev A, Schultze P, Wörgötter E, Braun W, Wagner G, Vašák M, Kägi J H R, Wüthrich K. J Mol Biol. 1988;201:637–657. doi: 10.1016/0022-2836(88)90644-4. [DOI] [PubMed] [Google Scholar]
- 34.Li T-Y, Minkel D T, Shaw C F, III, Petering D H. Biochem J. 1981;193:441–446. doi: 10.1042/bj1930441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savas M M, Petering D H, Shaw C F., III Inorg Chem. 1991;30:581–583. [Google Scholar]