Abstract
Mycoplasma gallisepticum antigens were prepared from organisms cultured in broth medium with glucose. The influence of period of growth, pH of the medium and duration of incubation at low pH (5·0) on the sensitivity of these antigens was determined in certain tests. The most sensitive antigen for the serum plate test was harvested after no more than 8 hr. incubation at pH 5·0. Sensitivity in serum plate, haemagglutination and gel diffusion tests was impaired if organisms were incubated at pH 5·0 for long periods. Antigens prepared from buffered broth medium were found to be at least as sensitive as those from unbuffered medium for the haemagglutination and gel precipitation tests, but considerably less so for the serum plate test.
Full text
PDF


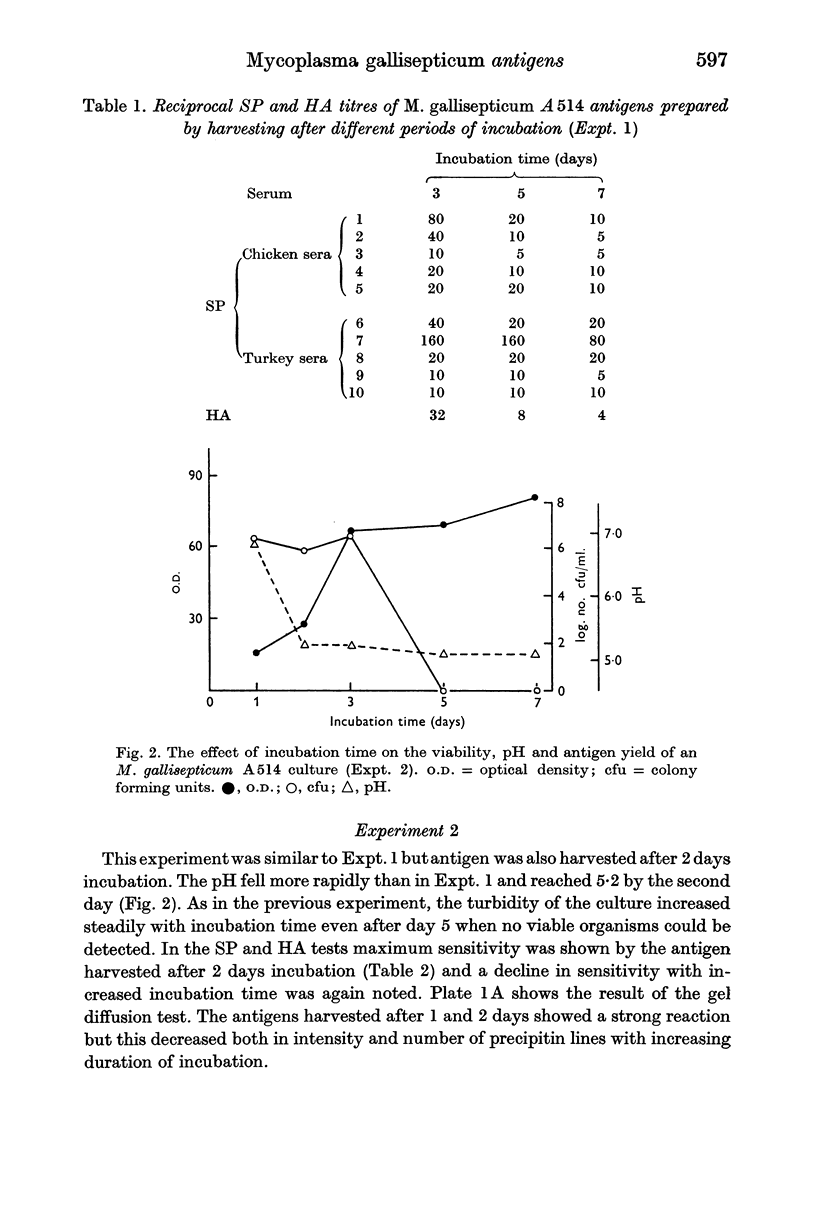
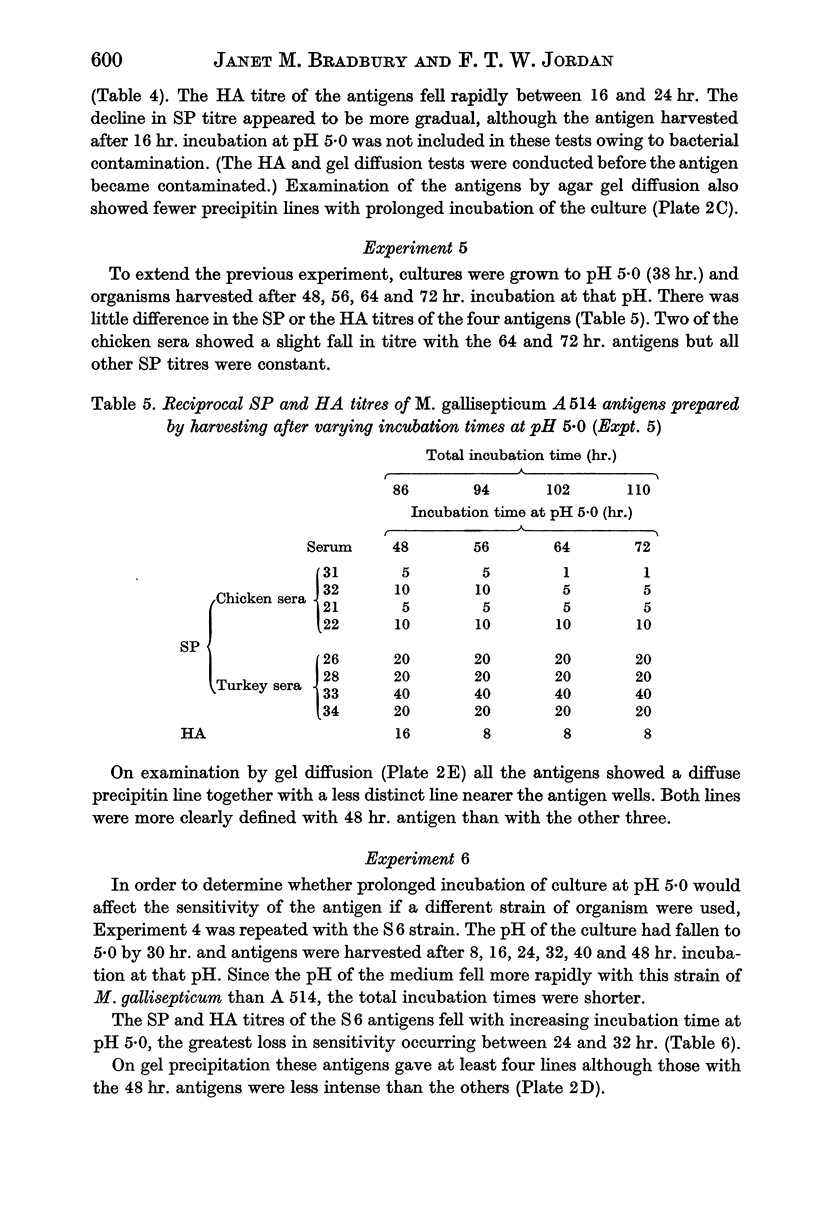





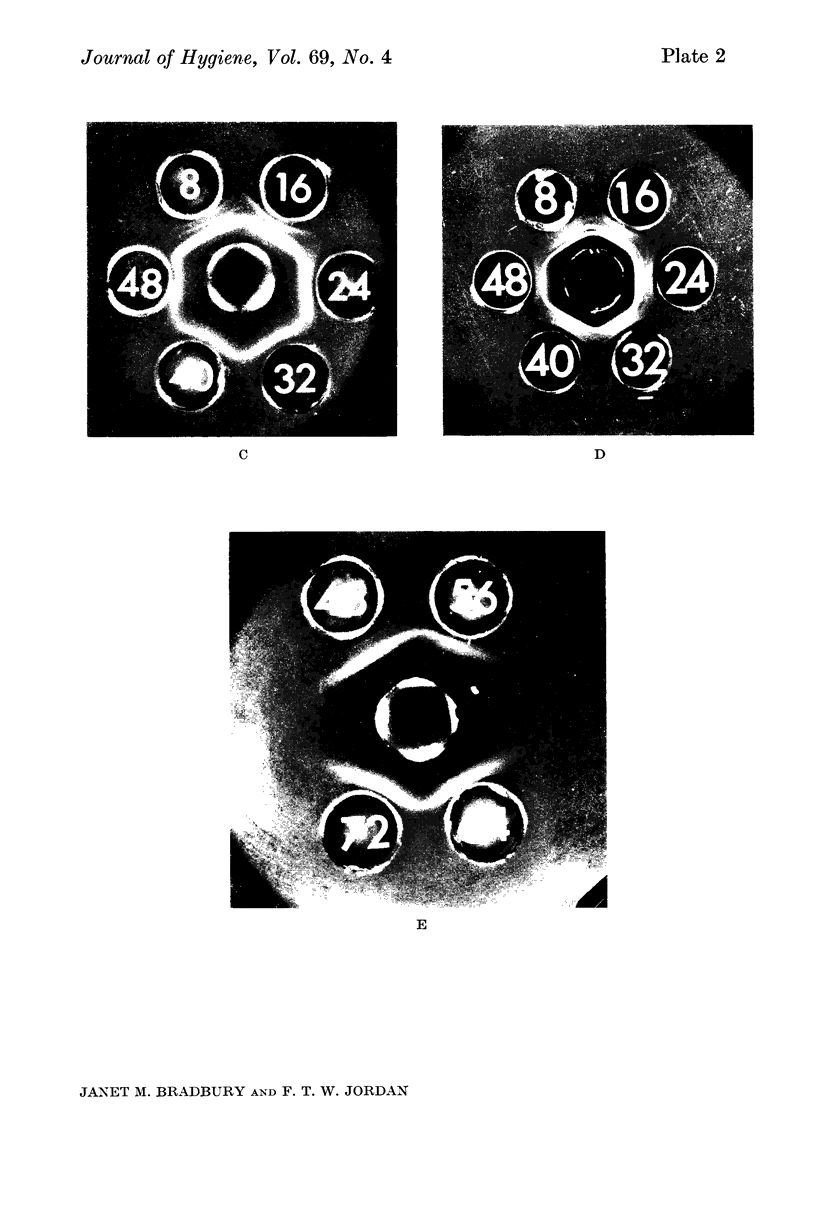
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. E., DaMassa A. J. Effect of dextrose in medium for the preparation of Mycoplasma gallisepticum plate antigens. Appl Microbiol. 1968 Apr;16(4):558–562. doi: 10.1128/am.16.4.558-562.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury J. M., Jordan F. T. Investigation into rabbit infusion media for the growth of Mycoplasma gallisepticum antigens for inoculation into rabbits. J Hyg (Lond) 1971 Mar;69(1):73–82. doi: 10.1017/s0022172400021264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng J. The complement fixation test with Mycoplasma pneumoniae antigen. 3. Observations on the development of complement fixing antigen in broth culture. Acta Pathol Microbiol Scand. 1969;75(4):598–610. [PubMed] [Google Scholar]
- Hromatka L., Adler H. E. Effects of pH, physical factors, and preservatives on the sensitivity of Mycoplasma gallisepticum slide agglutination antigens. Avian Dis. 1969 Aug;13(3):452–461. [PubMed] [Google Scholar]
- Jordan F. T., Kulasegaram P. Non-specific antibodies in chickens inoculated intratracheally with Mycoplasma gallisepticum. J Comp Pathol. 1968 Oct;78(4):407–414. doi: 10.1016/0021-9975(68)90038-8. [DOI] [PubMed] [Google Scholar]
- Jordan F. T., Kulasegaram P. Serological tests for the detection of antibodies to Mycoplasma gallisepticum in chickens and turkeys. J Hyg (Lond) 1968 Jun;66(2):249–267. doi: 10.1017/s0022172400041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach R. H., Blaxland J. D. The need for standardisation of serological techniques for the detection of Mycoplasma gallisepticum infection in poultry. Vet Rec. 1966 Sep 10;79(11):308–312. doi: 10.1136/vr.79.11.308. [DOI] [PubMed] [Google Scholar]
- Mayeda B., Lewis R. V. The effect of incubation time longer than 24 hours on Mycoplasma gallisepticum serum tube agglutination test titers. Avian Dis. 1969 Nov;13(4):783–792. [PubMed] [Google Scholar]
- Pollack J. D., Somerson N. L., Senterfit L. B. Effect of pH on the immunogenicity of Mycoplasma pneumoniae. J Bacteriol. 1969 Feb;97(2):612–619. doi: 10.1128/jb.97.2.612-619.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. H. Non-specific agglutination reactions with Mycoplasma gallisepticum antigens. Vet Rec. 1970 Aug 1;87(5):125–126. doi: 10.1136/vr.87.5.125. [DOI] [PubMed] [Google Scholar]
- Roberts D. H., Olesiuk O. M., Van Roekel H. Immunologic response of fowl to Mycoplasma gallisepticum and its relationship to latent infection. Am J Vet Res. 1967 Jul;28(125):1135–1152. [PubMed] [Google Scholar]







