Abstract
The use of plastic isolators and of an `air curtain' isolator for protection of patients against infection was studied in a burns unit.
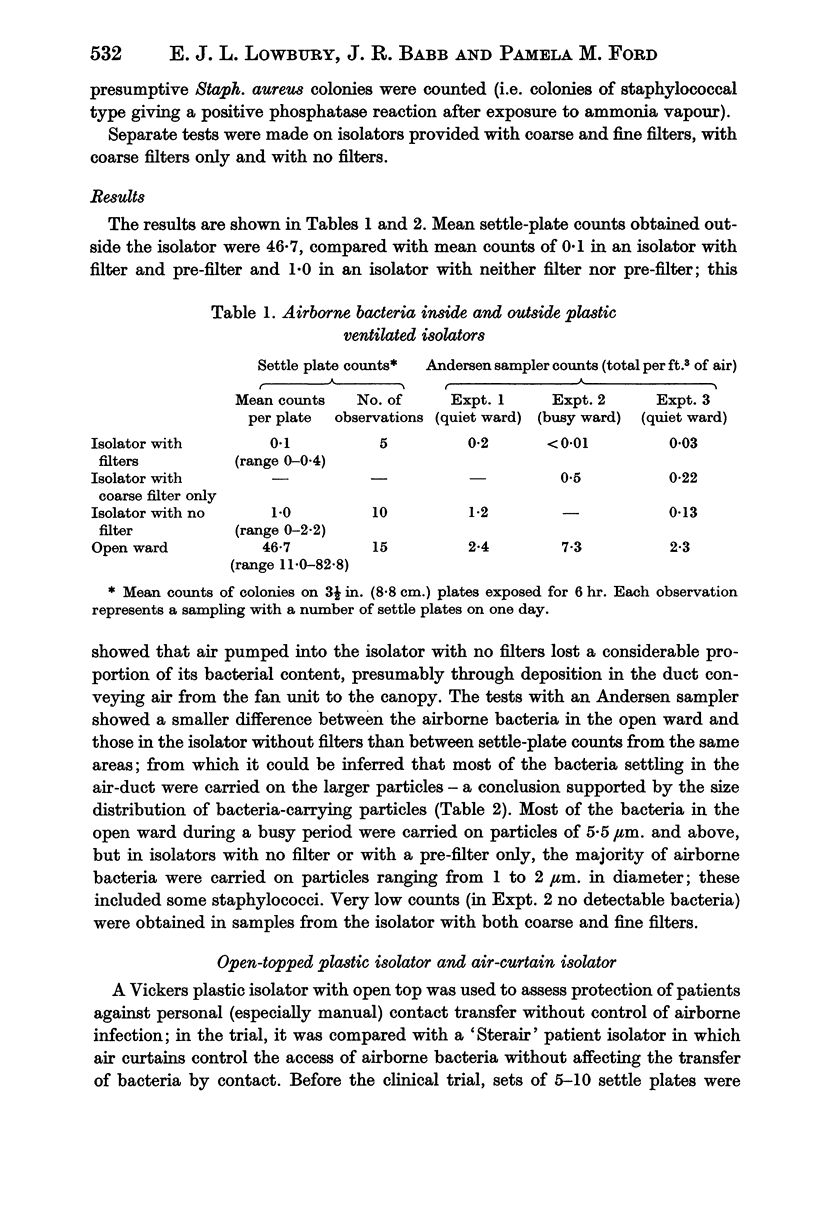
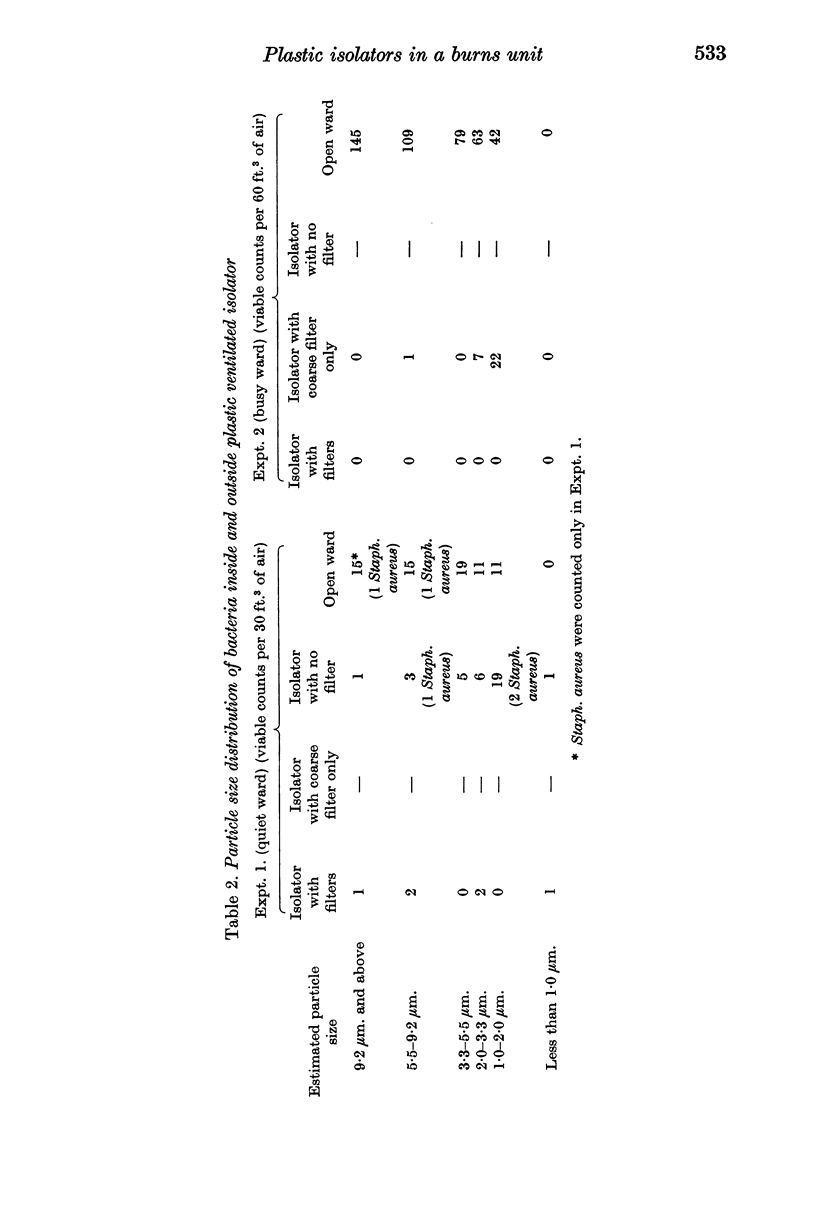
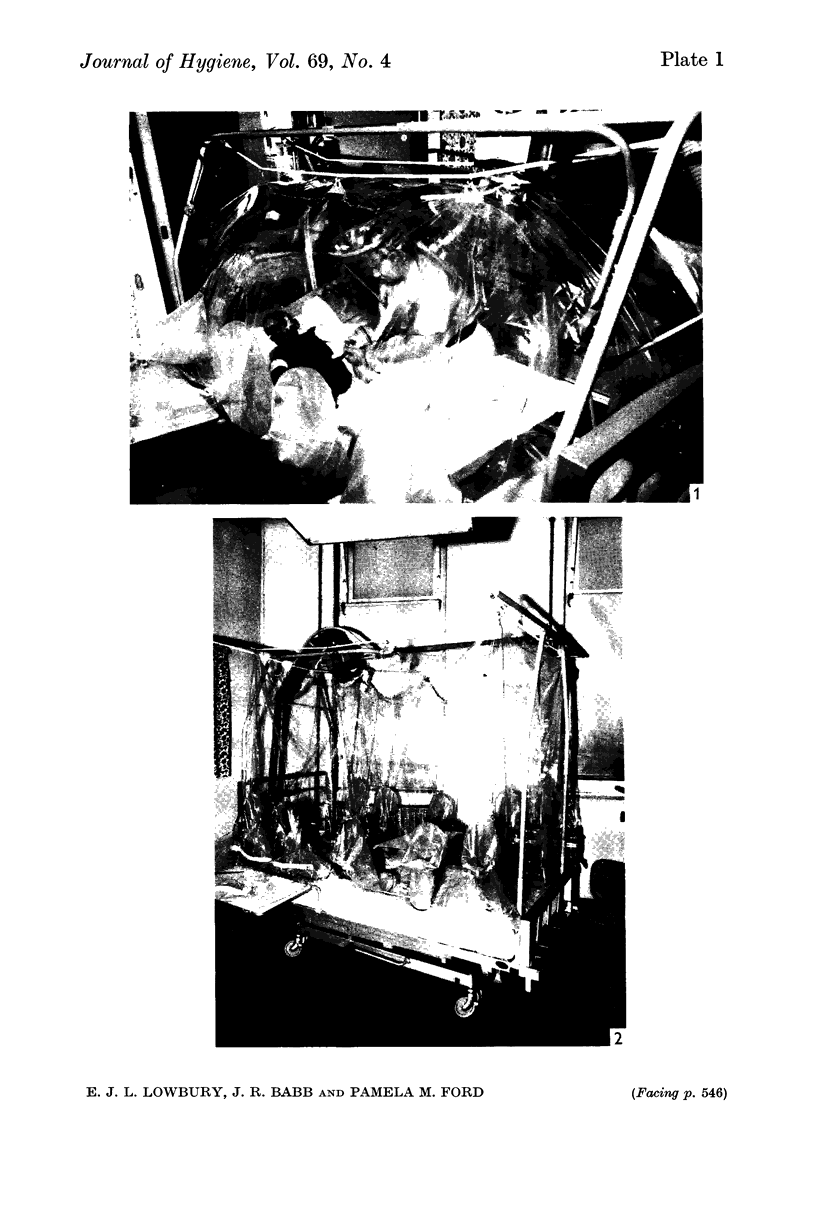
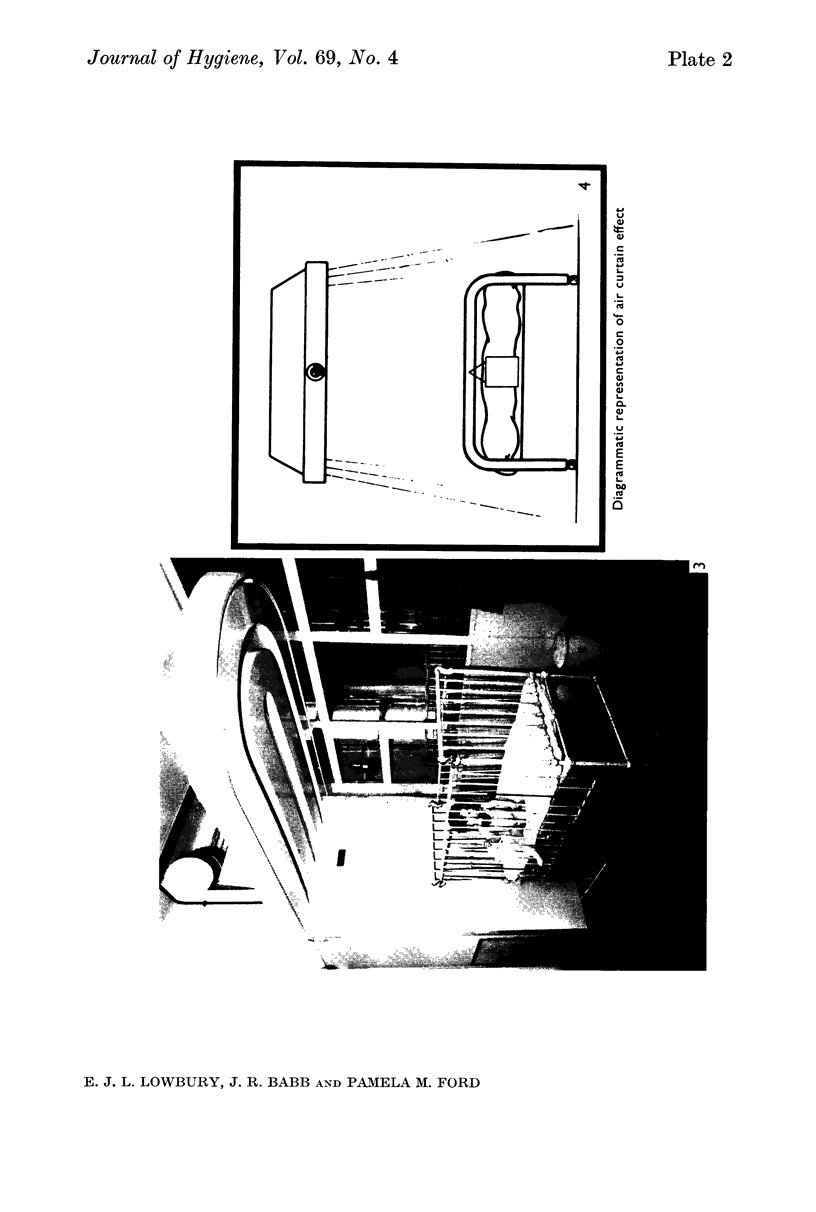
Preliminary bacteriological tests showed that very few airborne bacteria gained access to a plastic ventilated isolator; even when the filter and pre-filter were removed from the air inflow, settle-plate counts inside the isolator were much lower than those in the open ward, but the difference was smaller in tests made with an Anderson air sampler, which showed also that fewer large bacteria-carrying particles appeared inside the isolator than outside it. An open-topped isolator allowed virtually free access of bacteria from ambient air. The numbers of airborne bacteria inside an air curtain were appreciably lower than the counts of airborne bacteria in the open ward, but not as low as those in the plastic ventilated isolator.
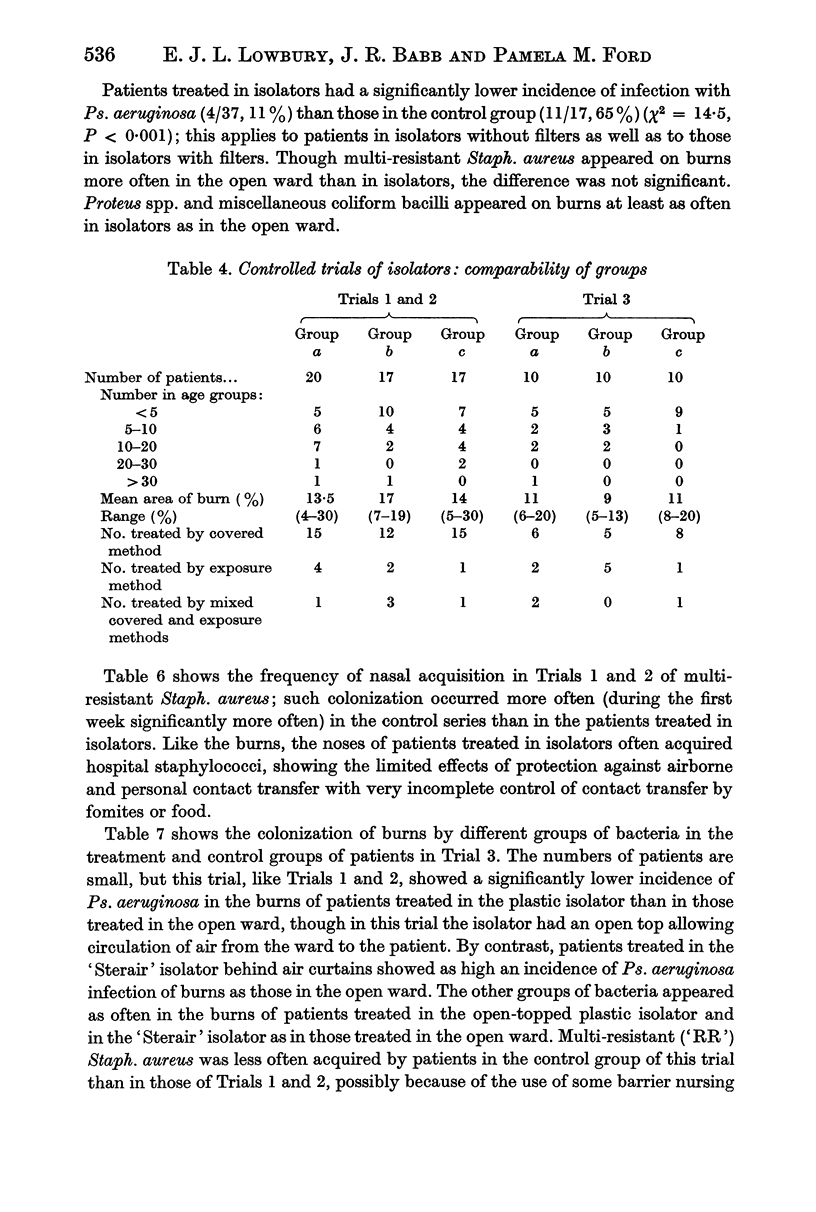
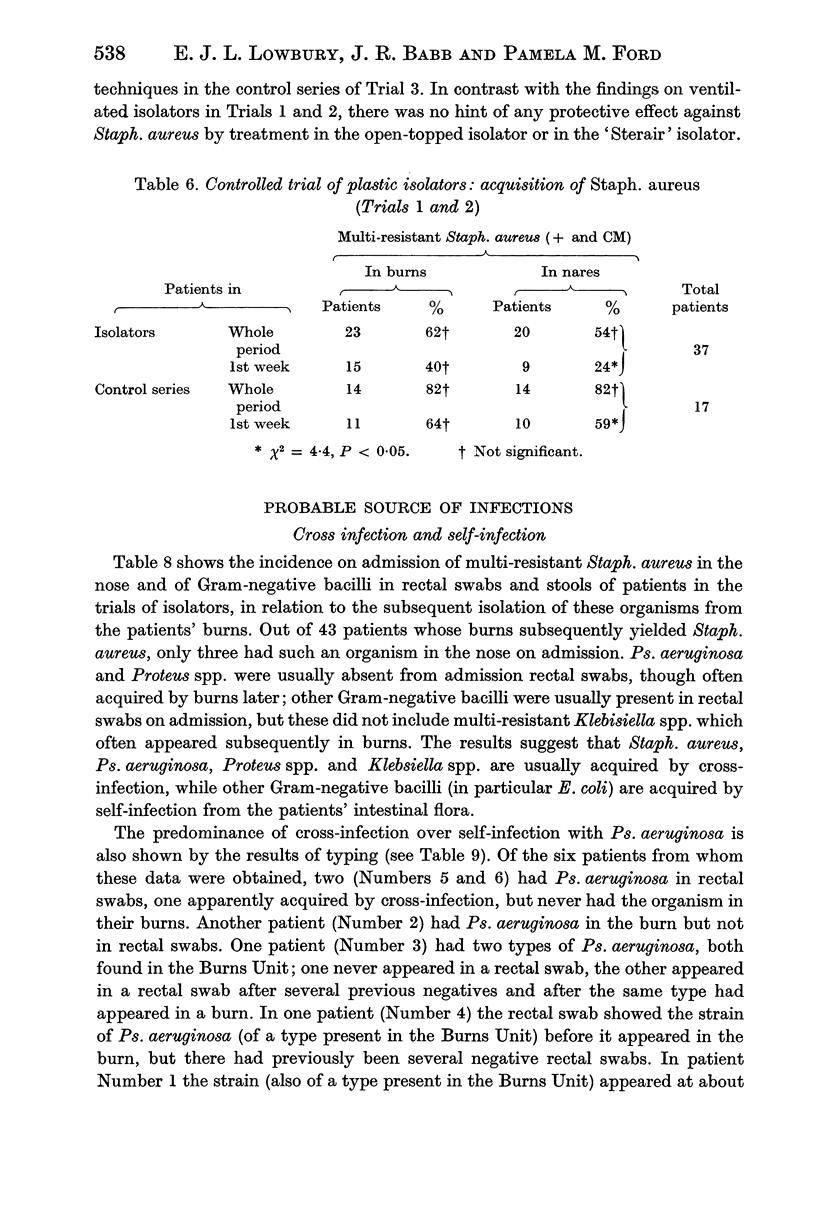
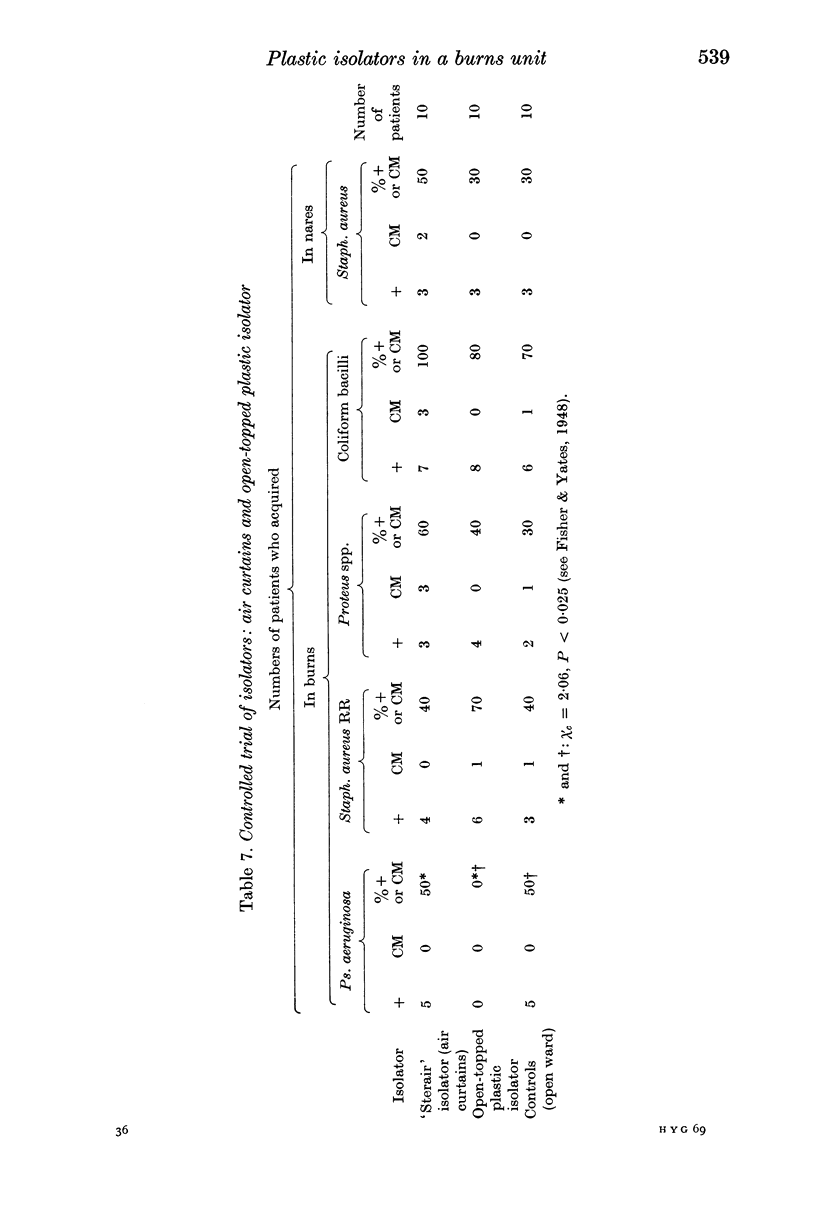
Controlled trials of isolators were made on patients with fresh burns of 4-30% of the body surface; the patients were given no topical chemoprophylaxis against Staphylococcus aureus or Gram-negative bacilli. Patients treated in plastic isolators showed a significantly lower incidence of infection with Pseudomonas aeruginosa than those treated in the open ward; this protective effect was shown by isolators with or without filters or with an open top. Ventilated isolators, which protected patients against personal contact and airborne infection, gave a limited protection against multi-resistant `hospital' strains of Staph. aureus, but no such protection was given by an open-topped isolator, which protected only against personal contact infection, or by air curtains, which protected only against airborne infection; the air curtain gave no protection against Ps. aeruginosa, and there was no evidence of protection by any isolator against Proteus spp. and coliform bacilli.
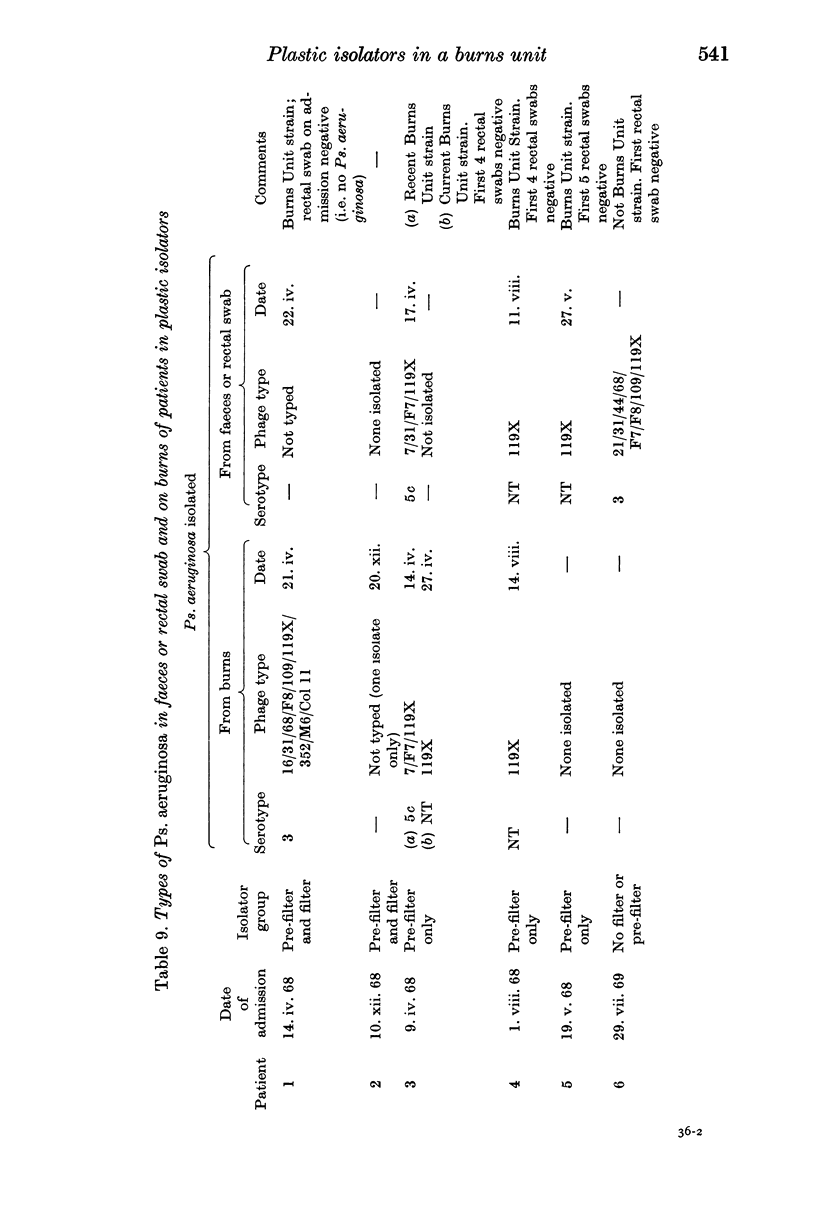
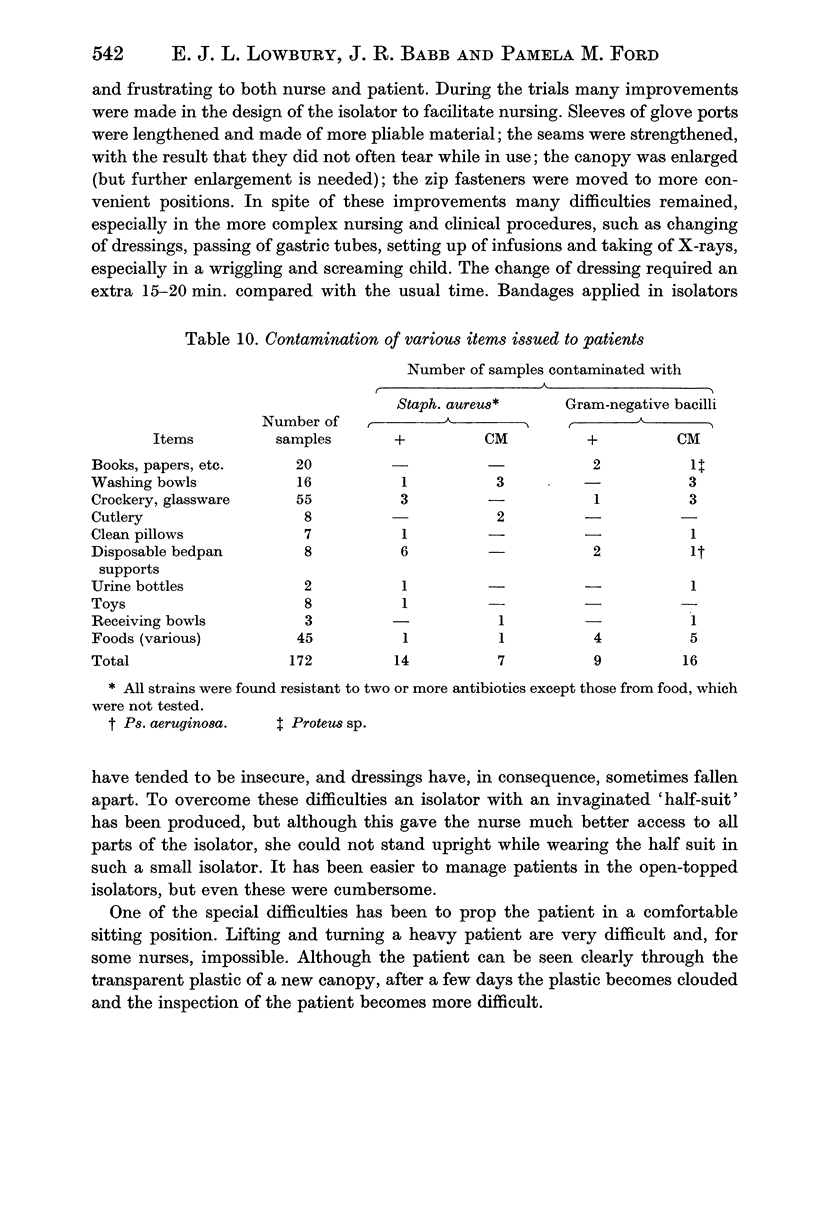
Both the controlled trials and evidence from the bacteriology of air, hands, fomites and rectal and nasal swabs taken on admission and later, supported the view that Ps. aeruginosa is transferred mainly by personal contact, Staph. aureus probably by air as well as by contact and coliform bacilli mainly by self infection with faecal flora, many of which are first acquired from the hospital environment in food or on fomites.
The use of plastic isolators is cumbersome, and of limited value except in the control of infection with Ps. aeruginosa. For this reason and because of the effectiveness of topical chemoprophylaxis such isolators are unlikely to have more than an occasional use in the treatment of burns. Though air curtains greatly reduce airborne contamination, their use in a burns unit does not appear to protect patients against infection when the alternative (and, for Ps. aeruginosa, more important) routes of contamination by personal contact and fomites are left open.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayliffe G. A., Collins B. J., Lowbury E. J., Wall M. Protective isolation in single-bed rooms: studies in a modified hospital ward. J Hyg (Lond) 1971 Dec;69(4):511–527. doi: 10.1017/s0022172400021793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARBER M., KUPER S. W. A. Identification of Staphylococcus pyogenes by the phosphatase reaction. J Pathol Bacteriol. 1951 Jan;63(1):65–68. doi: 10.1002/path.1700630108. [DOI] [PubMed] [Google Scholar]
- Cason J. S., Jackson D. M., Lowbury E. J., Ricketts C. R. Antiseptic and aseptic prophylaxis for burns: use of silver nitrate and of isolators. Br Med J. 1966 Nov 26;2(5525):1288–1294. doi: 10.1136/bmj.2.5525.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B., Lilly H. A., Lowbury E. J. Gram-negative bacilli in burns. J Clin Pathol. 1969 Nov;22(6):634–641. doi: 10.1136/jcp.22.6.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVENSON S. M., TREXLER P. C., LACONTE M., PULASKI E. J. APPLICATION OF THE TECHNOLOGY OF THE GERMFREE LABORATORY TO SPECIAL PROBLEMS OF PATIENT CARE. Am J Surg. 1964 May;107:710–722. doi: 10.1016/0002-9610(64)90298-3. [DOI] [PubMed] [Google Scholar]
- LOWBURY E. J. Air-conditioning with filtered air for dressing burns. Lancet. 1954 Feb 6;266(6806):292–294. doi: 10.1016/s0140-6736(54)91036-3. [DOI] [PubMed] [Google Scholar]
- LOWBURY E. J., FOX J. The epidemiology of infection with Pseudomonas pyocyanea in a burns unit. J Hyg (Lond) 1954 Sep;52(3):403–416. doi: 10.1017/s0022172400027601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWBURY E. J. Infection of burns. Br Med J. 1960 Apr 2;1(5178):994–1001. doi: 10.1136/bmj.1.5178.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidwell O. M., Towers A. G. Protection from microbial contamination in a room ventilated by a uni-directional air flow. J Hyg (Lond) 1969 Mar;67(1):95–106. doi: 10.1017/s0022172400041474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOPLEY E., LOWBURY E. J. L., HURST L. Bacteriological control of aureomycin therapy. Lancet. 1951 Jan 13;1(6646):87–89. doi: 10.1016/s0140-6736(51)91169-5. [DOI] [PubMed] [Google Scholar]