Abstract
Uncoupled enzyme IIGlc of the phosphoenolpyruvate (PEP):glucose phosphotransferase system (PTS) in Salmonella typhimurium is able to catalyze glucose transport in the absence of PEP-dependent phosphorylation. As a result of the ptsG mutation, the apparent Km of the system for glucose transport is increased about 1,000-fold (approximately 18 mM) compared with wild-type PTS-mediated glucose transport. An S. typhimurium mutant containing uncoupled enzyme IIGlc as the sole system for glucose uptake was grown in glucose-limited chemostat cultures. Selective pressure during growth in the chemostat resulted in adaptation to the glucose-limiting conditions in two different ways. At first, mutations appeared that led to a decrease in Km value of uncoupled enzyme IIGlc. These results suggested that uncoupled enzyme IIGlc had significant control on the growth rate under glucose-limiting conditions. More efficient glucose uptake enabled a mutant to outgrow its parent and caused a decrease in the steady-state glucose concentration in the chemostat. At very low glucose concentrations (10 microM), mutants arose that contained a constitutively synthesized methyl-beta-galactoside permease. Apparently, further changes in the uncoupled enzyme IIGlc did not lead to a substantial increase in growth rate at very low glucose concentrations.
Full text
PDF
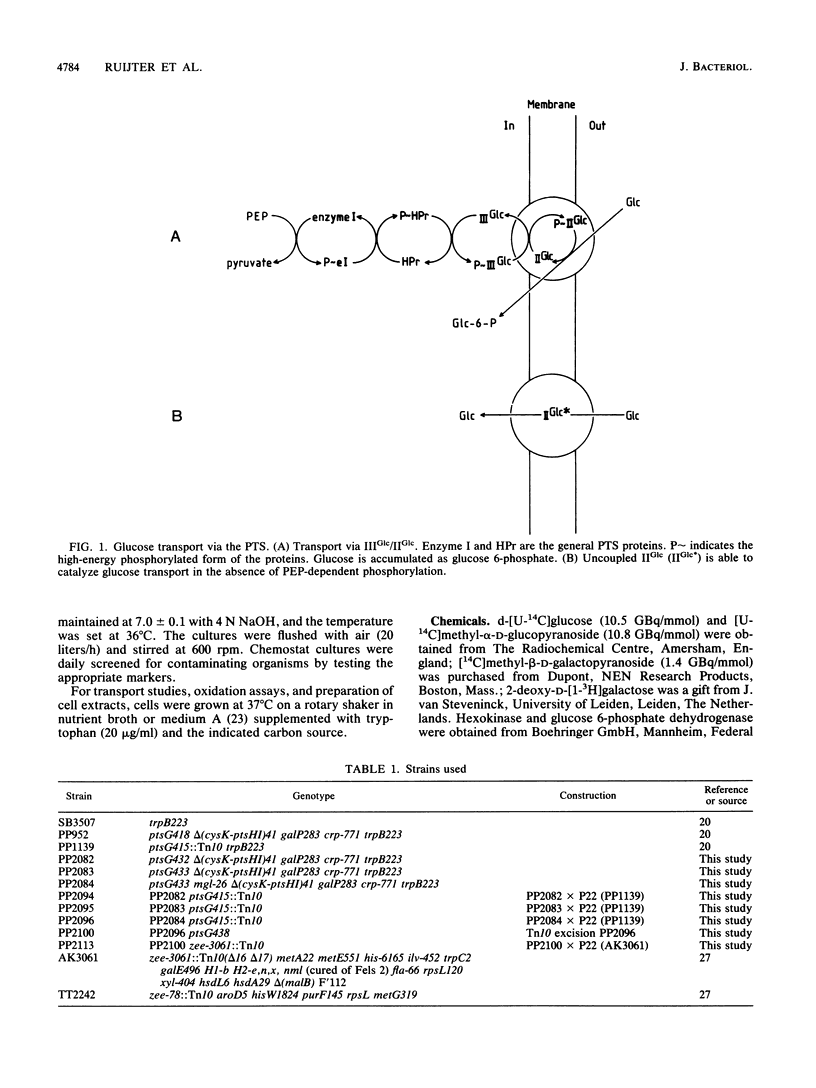





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner-Luger D., Boos W. The mglB sequence of Salmonella typhimurium LT2; promoter analysis by gene fusions and evidence for a divergently oriented gene coding for the mgl repressor. Mol Gen Genet. 1988 Nov;214(3):579–587. doi: 10.1007/BF00330498. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A. M., Dykhuizen D. E., Hartl D. L. Fitness as a function of beta-galactosidase activity in Escherichia coli. Genet Res. 1986 Aug;48(1):1–8. doi: 10.1017/s0016672300024587. [DOI] [PubMed] [Google Scholar]
- Dykhuizen D. E., Dean A. M., Hartl D. L. Metabolic flux and fitness. Genetics. 1987 Jan;115(1):25–31. doi: 10.1093/genetics/115.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen D. E., Hartl D. L. Selection in chemostats. Microbiol Rev. 1983 Jun;47(2):150–168. doi: 10.1128/mr.47.2.150-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Kuenen J. G. A review. Microbial selection in continuous culture. J Appl Bacteriol. 1977 Aug;43(1):1–24. doi: 10.1111/j.1365-2672.1977.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Hartman P. E. Some improved methods in P22 transduction. Genetics. 1974 Apr;76(4):625–631. doi: 10.1093/genetics/76.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Vargas C. N., Adams J. Evolution of Escherichia coli during growth in a constant environment. Genetics. 1987 Jul;116(3):349–358. doi: 10.1093/genetics/116.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Riordan C. Uptake of galactose into Escherichia coli by facilitated diffusion. J Gen Microbiol. 1976 May;94(1):75–89. doi: 10.1099/00221287-94-1-75. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meins M., Zanolari B., Rosenbusch J. P., Erni B. Glucose permease of Escherichia coli. Purification of the IIGlc subunit and functional characterization of its oligomeric forms. J Biol Chem. 1988 Sep 15;263(26):12986–12993. [PubMed] [Google Scholar]
- Nagelkerke F., Postma P. W. 2-deoxygalactose, a specific substrate of the Salmonella typhiimurium galactose permease: its use for the isolation of galP mutants. J Bacteriol. 1978 Feb;133(2):607–613. doi: 10.1128/jb.133.2.607-613.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Postma P. W. Defective enzyme II-BGlc of the phosphoenolpyruvate:sugar phosphotransferase system leading to uncoupling of transport and phosphorylation in Salmonella typhimurium. J Bacteriol. 1981 Aug;147(2):382–389. doi: 10.1128/jb.147.2.382-389.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W. Galactose transport in Salmonella typhimurium. J Bacteriol. 1977 Feb;129(2):630–639. doi: 10.1128/jb.129.2.630-639.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W. Involvement of the phosphotransferase system in galactose transport in Salmonella typhimurium. FEBS Lett. 1976 Jan 1;61(1):49–53. doi: 10.1016/0014-5793(76)80169-x. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Keizer H. G., Koolwijk P. Transport of trehalose in Salmonella typhimurium. J Bacteriol. 1986 Dec;168(3):1107–1111. doi: 10.1128/jb.168.3.1107-1111.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Stock J. B. Enzymes II of the phosphotransferase system do not catalyze sugar transport in the absence of phosphorylation. J Bacteriol. 1980 Feb;141(2):476–484. doi: 10.1128/jb.141.2.476-484.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Kinetic analyses of the sugar phosphate:sugar transphosphorylation reaction catalyzed by the glucose enzyme II complex of the bacterial phosphotransferase system. J Biol Chem. 1978 Nov 10;253(21):7595–7597. [PubMed] [Google Scholar]
- Robillard G. T., Beechey R. B. Evidence for the existence of a channel in the glucose-specific carrier EIIGlc of the Salmonella typhimurium phosphoenolpyruvate-dependent phosphotransferase system. Biochemistry. 1986 Mar 25;25(6):1346–1354. doi: 10.1021/bi00354a024. [DOI] [PubMed] [Google Scholar]
- Rutgers M., Teixeira de Mattos M. J., Postma P. W., Van Dam K. Establishment of the steady state in glucose-limited chemostat cultures of Klebsiella pneumoniae. J Gen Microbiol. 1987 Feb;133(2):445–451. doi: 10.1099/00221287-133-2-445. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonti R. V., Roth J. R. Role of gene duplications in the adaptation of Salmonella typhimurium to growth on limiting carbon sources. Genetics. 1989 Sep;123(1):19–28. doi: 10.1093/genetics/123.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Waygood E. B., Meadow N. D., Postma P. W., Roseman S. Sugar transport by the bacterial phosphotransferase system. The glucose receptors of the Salmonella typhimurium phosphotransferase system. J Biol Chem. 1982 Dec 10;257(23):14543–14552. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]



