Abstract
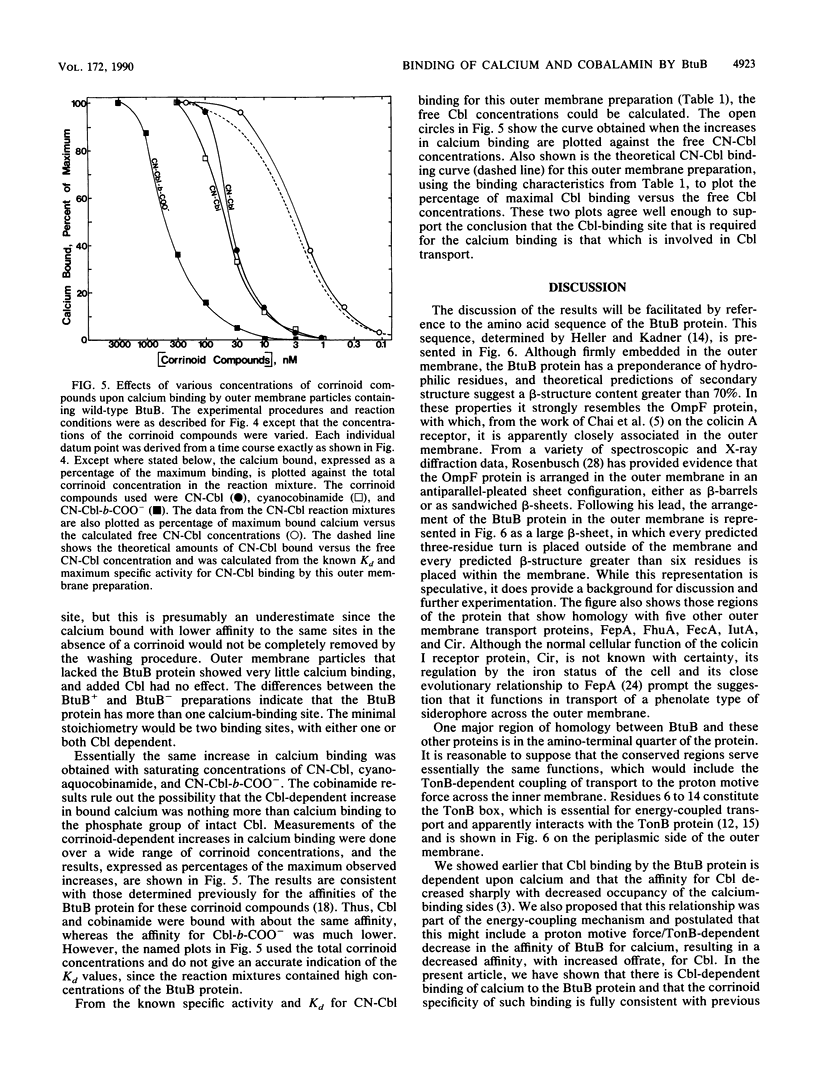
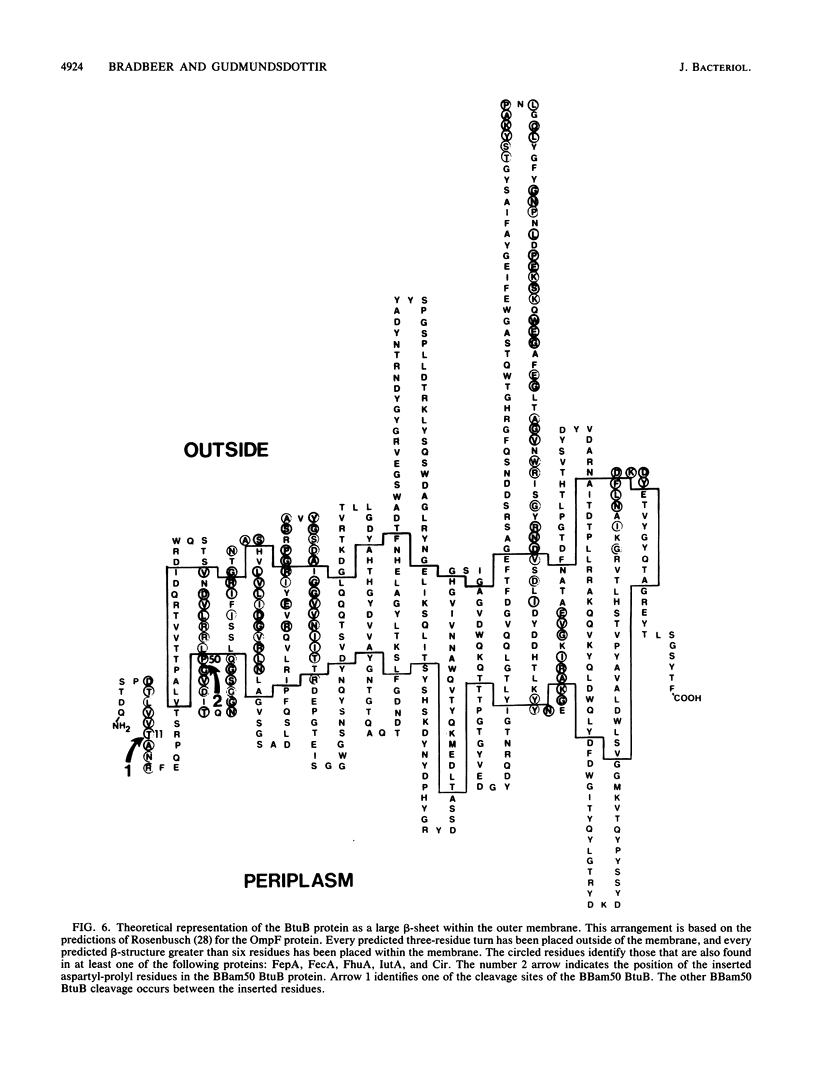
The binding of calcium and cobalamin to outer membranes from cells of Escherichia coli that contained amplified levels of wild-type or mutant btuB was studied. The mutant (BBam50) had an aspartyl-prolyl dipeptide inserted after the original 50th amino acid residue of the mature BtuB protein, which is within a region that shows extensive homology with the ferric siderophore receptors. This insertion resulted in cleavage of the BtuB in two places. The larger form retained the insertion but had lost 11 amino acid residues from the amino terminus. The smaller form was cut at the insertion site. Both the wild-type protein and the larger form of mutant BtuB showed calcium-dependent cobalamin binding with the same affinity for cobalamin, although the mutant had a much lower affinity for calcium. The smaller form of the mutant BtuB protein had a greatly reduced affinity for cobalamin, which was probably the result of inactivation of the cobalamin-dependent calcium-binding site. Cobalamin-dependent calcium binding was measured in wild-type BtuB preparations and was found to have the same corrinoid specificity and response to various corrinoid concentrations as shown previously for cobalamin binding. The results are consistent with a role for calcium in the cobalamin pump of the outer membrane of E. coli and show that a conserved part of the BtuB protein is required for the cobalamin-dependent binding of calcium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Teplow D. B., Hood L. E., Kent S. B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. J Biol Chem. 1986 Mar 25;261(9):4229–4238. [PubMed] [Google Scholar]
- Bassford P. J., Jr, Bradbeer C., Kadner R. J., Schnaitman C. A. Transport of vitamin B12 in tonB mutants of Escherichia coli. J Bacteriol. 1976 Oct;128(1):242–247. doi: 10.1128/jb.128.1.242-247.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbeer C., Reynolds P. R., Bauler G. M., Fernandez M. T. A requirement for calcium in the transport of cobalamin across the outer membrane of Escherichia coli. J Biol Chem. 1986 Feb 25;261(6):2520–2523. [PubMed] [Google Scholar]
- Bradbeer C., Woodrow M. L., Khalifah L. I. Transport of vitamin B12 in Escherichia coli: common receptor system for vitamin B12 and bacteriophage BF23 on the outer membrane of the cell envelope. J Bacteriol. 1976 Mar;125(3):1032–1039. doi: 10.1128/jb.125.3.1032-1039.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai T., Wu V., Foulds J. Colicin A receptor: role of two Escherichia coli outer membrane proteins (OmpF protein and btuB gene product) and lipopolysaccharide. J Bacteriol. 1982 Aug;151(2):983–988. doi: 10.1128/jb.151.2.983-988.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Cameron D. R., Carmel G., Jean R., Rode H. N. Protein fusions of beta-galactosidase to the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1986 Jan;165(1):181–192. doi: 10.1128/jb.165.1.181-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Masi D. R., White J. C., Schnaitman C. A., Bradbeer C. Transport of vitamin B12 in Escherichia coli: common receptor sites for vitamin B12 and the E colicins on the outer membrane of the cell envelope. J Bacteriol. 1973 Aug;115(2):506–513. doi: 10.1128/jb.115.2.506-513.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. Relationship between the tonB locus and iron transport in Escherichia coli. J Bacteriol. 1975 Nov;124(2):704–712. doi: 10.1128/jb.124.2.704-712.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsdottir A., Bell P. E., Lundrigan M. D., Bradbeer C., Kadner R. J. Point mutations in a conserved region (TonB box) of Escherichia coli outer membrane protein BtuB affect vitamin B12 transport. J Bacteriol. 1989 Dec;171(12):6526–6533. doi: 10.1128/jb.171.12.6526-6533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsdottir A., Bradbeer C., Kadner R. J. Altered binding and transport of vitamin B12 resulting from insertion mutations in the Escherichia coli btuB gene. J Biol Chem. 1988 Oct 5;263(28):14224–14230. [PubMed] [Google Scholar]
- Guterman S. K., Dann L. Excretion of enterochelin by exbA and exbB mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1225–1230. doi: 10.1128/jb.114.3.1225-1230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K. J., Kadner R. J., Günther K. Suppression of the btuB451 mutation by mutations in the tonB gene suggests a direct interaction between TonB and TonB-dependent receptor proteins in the outer membrane of Escherichia coli. Gene. 1988 Apr 15;64(1):147–153. doi: 10.1016/0378-1119(88)90488-x. [DOI] [PubMed] [Google Scholar]
- Heller K., Kadner R. J. Nucleotide sequence of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985 Mar;161(3):904–908. doi: 10.1128/jb.161.3.904-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., McElhaney G. Outer membrane-dependent transport systems in Escherichia coli: turnover of TonB function. J Bacteriol. 1978 Jun;134(3):1020–1029. doi: 10.1128/jb.134.3.1020-1029.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenley J. S., Leighton M., Bradbeer C. Transport of vitamin B12 in Escherichia coli. Corrinoid specificity of the outer membrane receptor. J Biol Chem. 1978 Mar 10;253(5):1341–1346. [PubMed] [Google Scholar]
- Konisky J., Soucek S., Frick K., Davies J. K., Hammond C. Relationship between the transport of iron and the amount of specific colicin Ia membrane receptors in Escherichia coli. J Bacteriol. 1976 Jul;127(1):249–257. doi: 10.1128/jb.127.1.249-257.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lundrigan M. D., Kadner R. J. Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli. Homology among outer membrane receptors that interact with TonB. J Biol Chem. 1986 Aug 15;261(23):10797–10801. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Nau C. D., Konisky J. Evolutionary relationship between the TonB-dependent outer membrane transport proteins: nucleotide and amino acid sequences of the Escherichia coli colicin I receptor gene. J Bacteriol. 1989 Feb;171(2):1041–1047. doi: 10.1128/jb.171.2.1041-1047.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Pressler U., Staudenmaier H., Zimmermann L., Braun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1988 Jun;170(6):2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. R., Mottur G. P., Bradbeer C. Transport of vitamin B12 in Escherichia coli. Some observations on the roles of the gene products of BtuC and TonB. J Biol Chem. 1980 May 10;255(9):4313–4319. [PubMed] [Google Scholar]
- Schnaitman C. A., McDonald G. A. Regulation of outer membrane protein synthesis in Escherichia coli K-12: deletion of ompC affects expression of the OmpF protein. J Bacteriol. 1984 Aug;159(2):555–563. doi: 10.1128/jb.159.2.555-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagegg W., Braun V. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein fecA. J Bacteriol. 1981 Jan;145(1):156–163. doi: 10.1128/jb.145.1.156-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- Weber G. Energetics of ligand binding to proteins. Adv Protein Chem. 1975;29:1–83. doi: 10.1016/s0065-3233(08)60410-6. [DOI] [PubMed] [Google Scholar]
- White J. C., DiGirolamo P. M., Fu M. L., Preston Y. A., Bradbeer C. Transport of vitamin B 12 in Escherichia coli. Location and properties of the initial B 12 -binding site. J Biol Chem. 1973 Jun 10;248(11):3978–3986. [PubMed] [Google Scholar]