Abstract
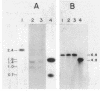
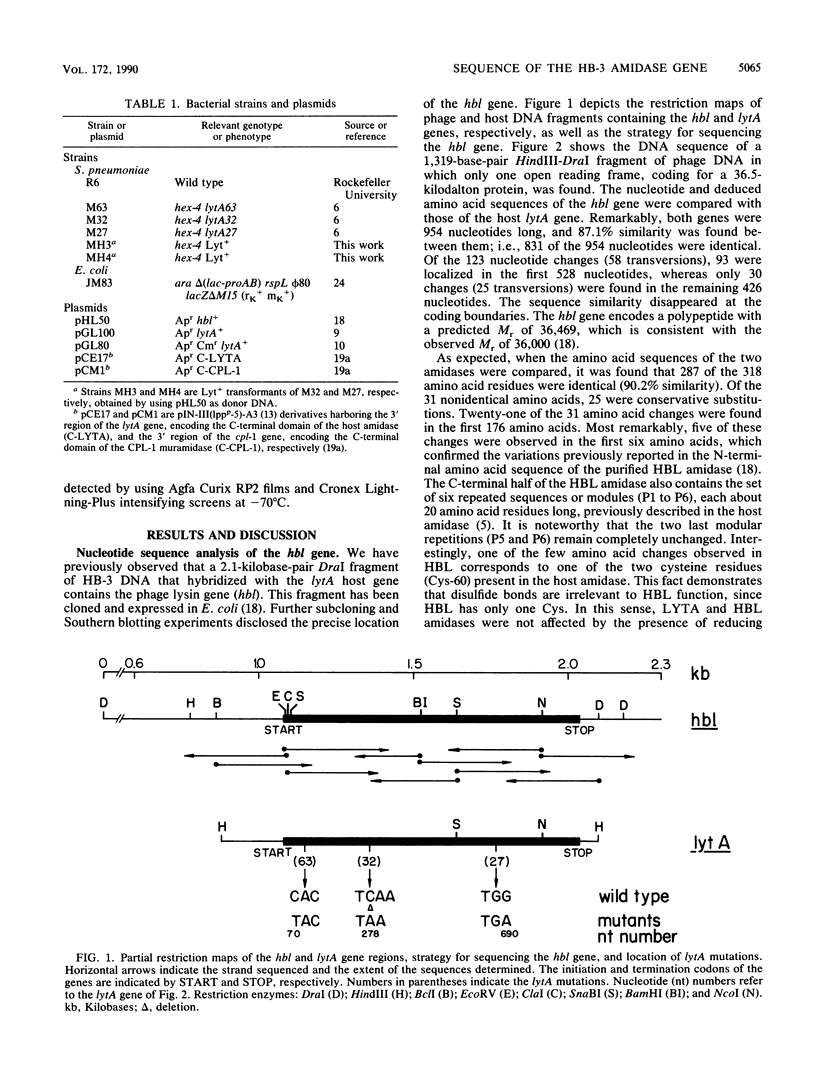
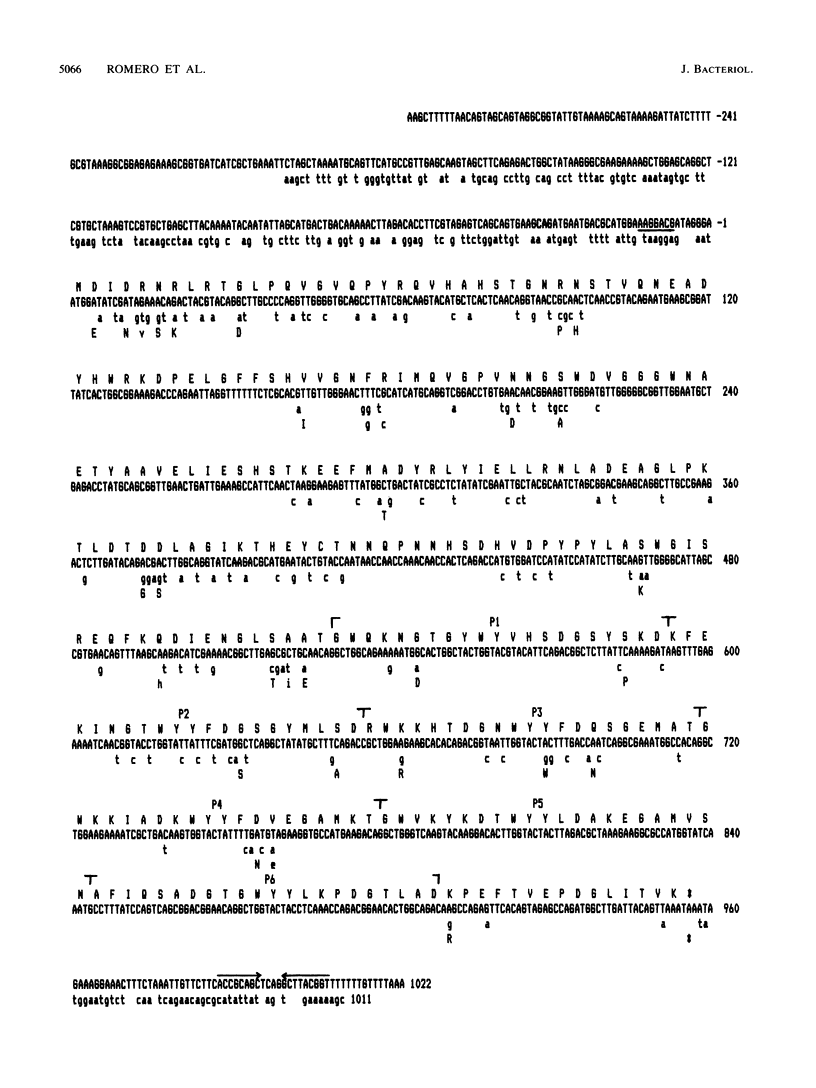
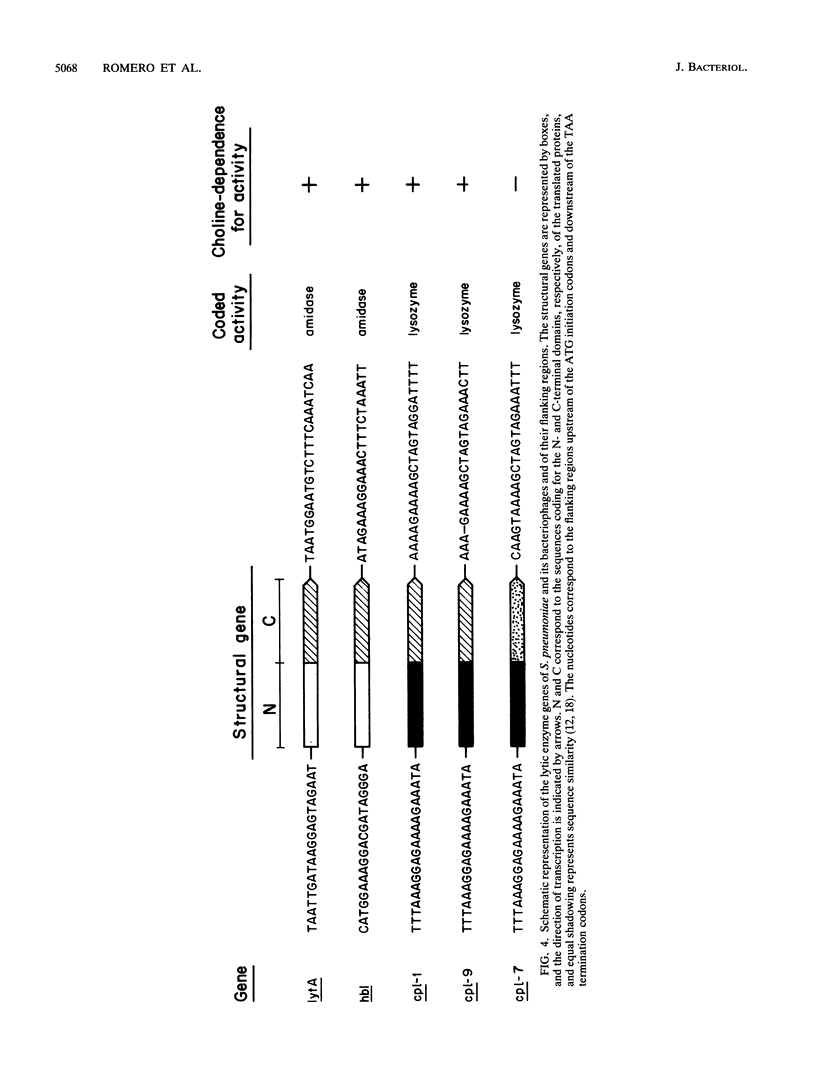
We have sequenced a DNA fragment containing the pneumococcal bacteriophage HB-3 hbl gene, which codes for the phage lytic amidase. A remarkable nucleotide similarity (87.1%) between the lytA gene, coding for the pneumococcal amidase, the major autolysin of Streptococcus pneumoniae, and the hbl gene was found. This similarity completely disappeared outside the open reading frames coding for both amidases. The hbl gene transformed amidase-deficient strains of S. pneumoniae to the wild-type phenotype, and Southern blotting experiments provided evidence for recombination between donor and recipient genes. A comprehensive evaluation of these and previous results on the peptidoglycan hydrolases of S. pneumoniae and its bacteriophages suggested that recombination mechanisms participate in the evolution of the genes coding for these enzymes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernheimer H. P. Lysogenic pneumococci and their bacteriophages. J Bacteriol. 1979 May;138(2):618–624. doi: 10.1128/jb.138.2.618-624.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. L., Nishida T., Kawamukai M., Utsumi R., Sakai H., Komano T. Cloning and sequencing of an Escherichia coli gene, nlp, highly homologous to the ner genes of bacteriophages Mu and D108. J Bacteriol. 1989 Sep;171(9):5222–5225. doi: 10.1128/jb.171.9.5222-5225.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz E., García E., Ascaso C., Méndez E., López R., García J. L. Subcellular localization of the major pneumococcal autolysin: a peculiar mechanism of secretion in Escherichia coli. J Biol Chem. 1989 Jan 15;264(2):1238–1244. [PubMed] [Google Scholar]
- Ellis H. B. The Use of Benzoylvinyl-Diacetone-Alkamine Lactate. Cal State J Med. 1905 May;3(5):142–143. [PMC free article] [PubMed] [Google Scholar]
- Garcia P., Garcia E., Ronda C., Lopez R., Tomasz A. A phage-associated murein hydrolase in Streptococcus pneumoniae infected with bacteriophage Dp-1. J Gen Microbiol. 1983 Feb;129(2):489–497. doi: 10.1099/00221287-129-2-489. [DOI] [PubMed] [Google Scholar]
- García E., García J. L., García P., Arrarás A., Sánchez-Puelles J. M., López R. Molecular evolution of lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Proc Natl Acad Sci U S A. 1988 Feb;85(3):914–918. doi: 10.1073/pnas.85.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García E., García J. L., Ronda C., García P., López R. Cloning and expression of the pneumococcal autolysin gene in Escherichia coli. Mol Gen Genet. 1985;201(2):225–230. doi: 10.1007/BF00425663. [DOI] [PubMed] [Google Scholar]
- García J. L., García E., López R. Overproduction and rapid purification of the amidase of Streptococcus pneumoniae. Arch Microbiol. 1987;149(1):52–56. doi: 10.1007/BF00423136. [DOI] [PubMed] [Google Scholar]
- García P., García J. L., García E., López R. Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promoter in Escherichia coli. Gene. 1986;43(3):265–272. doi: 10.1016/0378-1119(86)90215-5. [DOI] [PubMed] [Google Scholar]
- Inouye S., Inouye M. Up-promoter mutations in the lpp gene of Escherichia coli. Nucleic Acids Res. 1985 May 10;13(9):3101–3110. doi: 10.1093/nar/13.9.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P., Espinosa M., Stassi D. L., Lacks S. A. Facilitation of plasmid transfer in Streptococcus pneumoniae by chromosomal homology. J Bacteriol. 1982 May;150(2):692–701. doi: 10.1128/jb.150.2.692-701.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muesch A., Hartmann E., Rohde K., Rubartelli A., Sitia R., Rapoport T. A. A novel pathway for secretory proteins? Trends Biochem Sci. 1990 Mar;15(3):86–88. doi: 10.1016/0968-0004(90)90186-f. [DOI] [PubMed] [Google Scholar]
- Reanney D. C., Ackermann H. W. Comparative biology and evolution of bacteriophages. Adv Virus Res. 1982;27:205–280. doi: 10.1016/s0065-3527(08)60436-4. [DOI] [PubMed] [Google Scholar]
- Romero A., Lopez R., Garcia P. Characterization of the pneumococcal bacteriophage HB-3 amidase: cloning and expression in Escherichia coli. J Virol. 1990 Jan;64(1):137–142. doi: 10.1128/jvi.64.1.137-142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Puelles J. M., Ronda C., Garcia J. L., Garcia P., Lopez R., Garcia E. Searching for autolysin functions. Characterization of a pneumococcal mutant deleted in the lytA gene. Eur J Biochem. 1986 Jul 15;158(2):289–293. doi: 10.1111/j.1432-1033.1986.tb09749.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stroynowski I. T. Distribution of bacteriophage phi 3T homologous deoxyribonucleic acid sequences in Bacillus subtilis 168, related bacteriophages, and other Bacillus species. J Bacteriol. 1981 Oct;148(1):91–100. doi: 10.1128/jb.148.1.91-100.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Puelles J. M., Sanz J. M., García J. L., García E. Cloning and expression of gene fragments encoding the choline-binding domain of pneumococcal murein hydrolases. Gene. 1990 Apr 30;89(1):69–75. doi: 10.1016/0378-1119(90)90207-8. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]